The impact of vector-mediated neutrophil recruitment on cutaneous leishmaniasis
- PMID: 19545276
- PMCID: PMC3431610
- DOI: 10.1111/j.1462-5822.2009.01348.x
The impact of vector-mediated neutrophil recruitment on cutaneous leishmaniasis
Abstract
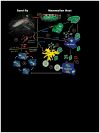
The dynamic process of pathogen transmission by the bite of an insect vector combines several biological processes that have undergone extensive co-evolution. Whereas the host response to an insect bite is only occasionally confronted with the parasitic pathogens that competent vectors might transmit, the transmitted parasites will always be confronted with the acute, wound-healing response that is initiated by the bite itself. Invariably, this response involves neutrophils. In the case of Leishmania, infection is initiated in the skin following the bite of an infected sand fly, suggesting that Leishmania must possess some means to survive their early encounter with recruited neutrophils at the bite site. Here, we review the literature regarding the impact of neutrophils on the outcome of infection with Leishmania, with special attention to the role of the sand fly bite.
Figures
References
-
- Afonso L, Borges VM, Cruz H, Ribeiro-Gomes FL, DosReis GA, Dutra AN, et al. Interactions with apoptotic but notwith necrotic neutrophils increase parasite burden in human macrophages infected with Leishmania amazonensis. J Leukoc Biol. 2008;84:389–396. - PubMed
-
- Aga E, Katschinski DM, van Zandbergen G, Laufs H, Hansen B, Muller K, et al. Inhibition of the spontaneous apoptosis of neutrophil granulocytes by the intracellular parasite Leishmania major. J Immunol. 2002;169:898–905. - PubMed
-
- al Tuwaijri AS, al Mofleh IA, Mahmoud AA. Effect of Leishmania major on human polymorphonuclear leucocyte function in vitro. J Med Microbiol. 1990;32:189–193. - PubMed
-
- Allen LA, McCaffrey RL. To activate or not to activate: distinct strategies used by Helicobacter pylori and Francisella tularensis to modulate the NADPH oxidase and survive in human neutrophils. Immunol Rev. 2007;219:103–117. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

