Can a nonequivalent choice of dosing regimen bias the results of flexible dose double blind trials? The CATIE schizophrenia trial
- PMID: 19545976
- PMCID: PMC6474782
- DOI: 10.1016/j.schres.2009.06.002
Can a nonequivalent choice of dosing regimen bias the results of flexible dose double blind trials? The CATIE schizophrenia trial
Abstract
Background: One of the major challenges in the design of double-blind flexible-dosing clinical trials comparing active drugs is the selection of dosing regimens that are equivalent across drugs. This study uses data from the CATIE schizophrenia trial to evaluate the hypothesis that drugs that were dosed somewhat higher in the trial than in typical practice would show greater efficacy and more side effects, especially at high capsule levels, than drugs that were dosed at lower relative strengths.
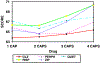
Methods: CATIE was a large (N=1460) randomized trial comparing 5 antipsychotics in patients with chronic schizophrenia. The blind was maintained in CATIE by prescribing identical-looking capsules of each medication. Dosing was flexible, such that PIs could prescribe from one to four capsules per day, and could modify the dose based on a patient's symptoms and side effects. Capsule strengths for olanzapine (7.5 mg) and quetiapine (200 mg) were relatively higher than for risperidone (1.5 mg), perphenazine (8 mg) or ziprasidone (40 mg). Proportional hazards models of time to all cause discontinuation and mixed regression models for continuous measures of symptoms, quality of life and side effects were used to test for interactions between randomly assigned drug and number of capsules prescribed per visit. We hypothesized that if a dosing bias was present, the flex-dosing design would result in a significant interaction such that drugs with higher relative dosing per capsule would be more effective and have more side effects than drugs with lower relative dosing and that this effect would be greatest at the largest prescribed dosing regimen (4 capsules).
Results: There were no significant interactions between drug assignment and number of capsules in the proportional hazards analyses of time to all cause discontinuation (p=.77, excluding ziprasidone and .74 in the ziprasidone cohort) or in the mixed model analysis of PANSS symptoms (p=.49), quality of life (p=.45); or measures of tardive dyskinesia (AIMS, p=.47). However a significant interaction was observed on the Barnes akathisia scale (p=.0005), on the Simpson Angus EPS scale (p=.10) and on the analysis of weight (p=0.014). Paired comparisons did not show the hypothesized pattern of relationships for akathisia or EPS, but such a pattern was suggested for olanzapine in the analysis of weight although it emerged at 2, 3 and 4 capsules indicating a general drug effect rather than a relative dosing difference.
Conclusion: Dosing biases do not seem to have affected the results of the CATIE trial.
Figures



References
-
- Barnes TRE, 1989. A rating scale for drug induced akathisia. Br J Psychiatry 131: 222–223. - PubMed
-
- Cox DR, 1972. Regression models and life tables. J R Stat Soc[B] 34: 187–220.
-
- First MB, Spitzer RL, Gibbon M, Williams JB, 1995. Structured Clinical Interview for DSM -IV ((SCID-I) (User’s Guide and Interview) Research Version. New York, NY, Biometrics Research Institute, New York State Psychiatric Institute.
-
- Guy W, 1976. Abnormal Involuntary Movements In: ECDEU Assessment Manual for Psychopharamcology, Guy W, ed. (DHEW No. ADM 76–338) Rockville, MD: National Institute of Mental Health.
-
- Heinrichs DW, Hanlon TE, Carpenter WT Jr., 1984. The Quality of Life Scale: an Instrument for rating the schizophrenic deficit syndrome. Schizophr Bull 10(3):388–98 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

