Neisseria gonorrhoeae infection protects human endocervical epithelial cells from apoptosis via expression of host antiapoptotic proteins
- PMID: 19546192
- PMCID: PMC2738021
- DOI: 10.1128/IAI.01366-08
Neisseria gonorrhoeae infection protects human endocervical epithelial cells from apoptosis via expression of host antiapoptotic proteins
Abstract
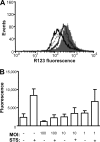
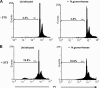
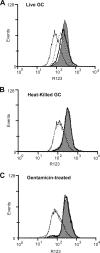
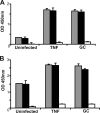
Several microbial pathogens can modulate the host apoptotic response to infection, which may contribute to immune evasion. Various studies have reported that infection with the sexually transmitted disease pathogen Neisseria gonorrhoeae can either inhibit or induce apoptosis. N. gonorrhoeae infection initiates at the mucosal epithelium, and in women, cells from the ectocervix and endocervix are among the first host cells encountered by this pathogen. In this study, we defined the antiapoptotic effect of N. gonorrhoeae infection in human endocervical epithelial cells (End/E6E7 cells). We first established that N. gonorrhoeae strain FA1090B failed to induce cell death in End/E6E7 cells. Subsequently, we demonstrated that stimulation with N. gonorrhoeae protected these cells from staurosporine (STS)-induced apoptosis. Importantly, only End/E6E7 cells incubated with live bacteria and in direct association with N. gonorrhoeae were protected from STS-induced apoptosis, while heat-killed and antibiotic-killed bacteria failed to induce protection. Stimulation of End/E6E7 cells with live N. gonorrhoeae induced NF-kappaB activation and resulted in increased gene expression of the NF-kappaB-regulated antiapoptotic genes bfl-1, cIAP-2, and c-FLIP. Furthermore, cIAP-2 protein levels also increased in End/E6E7 cells incubated with gonococci. Collectively, our results indicate that the antiapoptotic effect of N. gonorrhoeae in human endocervical epithelial cells results from live infection via expression of host antiapoptotic proteins. Securing an intracellular niche through the inhibition of apoptosis may be an important mechanism utilized by N. gonorrhoeae for microbial survival and immune evasion in cervical epithelial cells.
Figures

Similar articles
-
Neisseria gonorrhoeae Modulates Cell Death in Human Endocervical Epithelial Cells through Export of Exosome-Associated cIAP2.Infect Immun. 2015 Sep;83(9):3410-7. doi: 10.1128/IAI.00732-15. Epub 2015 Jun 15. Infect Immun. 2015. PMID: 26077759 Free PMC article.
-
Infection of human urethral epithelium with Neisseria gonorrhoeae elicits an upregulation of host anti-apoptotic factors and protects cells from staurosporine-induced apoptosis.Cell Microbiol. 2003 Aug;5(8):549-60. doi: 10.1046/j.1462-5822.2003.00300.x. Cell Microbiol. 2003. PMID: 12864814
-
Gonococcal porin IB activates NF-kappaB in human urethral epithelium and increases the expression of host antiapoptotic factors.Infect Immun. 2004 Nov;72(11):6408-17. doi: 10.1128/IAI.72.11.6408-6417.2004. Infect Immun. 2004. PMID: 15501771 Free PMC article.
-
The role of porins in neisserial pathogenesis and immunity.Trends Microbiol. 2003 Feb;11(2):87-93. doi: 10.1016/s0966-842x(02)00037-9. Trends Microbiol. 2003. PMID: 12598131 Review.
-
Regulation of TRAIL-induced apoptosis by ectopic expression of antiapoptotic factors.Vitam Horm. 2004;67:453-83. doi: 10.1016/S0083-6729(04)67023-3. Vitam Horm. 2004. PMID: 15110190 Review.
Cited by
-
Neisseria gonorrhoeae Modulates Cell Death in Human Endocervical Epithelial Cells through Export of Exosome-Associated cIAP2.Infect Immun. 2015 Sep;83(9):3410-7. doi: 10.1128/IAI.00732-15. Epub 2015 Jun 15. Infect Immun. 2015. PMID: 26077759 Free PMC article.
-
Neisseria gonorrhoeae-Induced Inflammatory Pyroptosis in Human Macrophages is Dependent on Intracellular Gonococci and Lipooligosaccharide.J Cell Death. 2018 Jan 3;11:1179066017750902. doi: 10.1177/1179066017750902. eCollection 2018. J Cell Death. 2018. PMID: 29434478 Free PMC article.
-
Restriction endonucleases from invasive Neisseria gonorrhoeae cause double-strand breaks and distort mitosis in epithelial cells during infection.PLoS One. 2014 Dec 2;9(12):e114208. doi: 10.1371/journal.pone.0114208. eCollection 2014. PLoS One. 2014. PMID: 25460012 Free PMC article.
-
Neisseria gonorrhoeae survives within and modulates apoptosis and inflammatory cytokine production of human macrophages.Cell Microbiol. 2016 Apr;18(4):546-60. doi: 10.1111/cmi.12529. Epub 2015 Oct 26. Cell Microbiol. 2016. PMID: 26426083 Free PMC article.
-
Crystallographic analysis of Neisseria meningitidis PorB extracellular loops potentially implicated in TLR2 recognition.J Struct Biol. 2014 Mar;185(3):440-7. doi: 10.1016/j.jsb.2013.12.006. Epub 2013 Dec 19. J Struct Biol. 2014. PMID: 24361688 Free PMC article.
References
-
- Airenne, S., H. M. Surcel, J. Tuukkanen, M. Leinonen, and P. Saikku. 2002. Chlamydia pneumoniae inhibits apoptosis in human epithelial and monocyte cell lines. Scand. J. Immunol. 55390-398. - PubMed
-
- Antonsson, B., and J. C. Martinou. 2000. The Bcl-2 protein family. Exp. Cell Res. 25650-57. - PubMed
-
- Ashkenazi, A., and V. M. Dixit. 1999. Apoptosis control by death and decoy receptors. Curr. Opin. Cell Biol. 11255-260. - PubMed
-
- Billker, O., A. Popp, S. D. Gray-Owen, and T. F. Meyer. 2000. The structural basis of CEACAM-receptor targeting by neisserial Opa proteins. Trends Microbiol. 8258-260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

