Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue
- PMID: 19546236
- PMCID: PMC2725728
- DOI: 10.1128/MCB.00192-09
Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue
Abstract
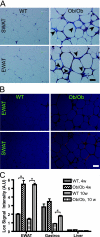
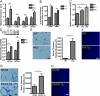
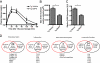
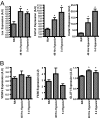
Adipose tissue can undergo rapid expansion during times of excess caloric intake. Like a rapidly expanding tumor mass, obese adipose tissue becomes hypoxic due to the inability of the vasculature to keep pace with tissue growth. Consequently, during the early stages of obesity, hypoxic conditions cause an increase in the level of hypoxia-inducible factor 1alpha (HIF1alpha) expression. Using a transgenic model of overexpression of a constitutively active form of HIF1alpha, we determined that HIF1alpha fails to induce the expected proangiogenic response. In contrast, we observed that HIF1alpha initiates adipose tissue fibrosis, with an associated increase in local inflammation. "Trichrome- and picrosirius red-positive streaks," enriched in fibrillar collagens, are a hallmark of adipose tissue suffering from the early stages of hypoxia-induced fibrosis. Lysyl oxidase (LOX) is a transcriptional target of HIF1alpha and acts by cross-linking collagen I and III to form the fibrillar collagen fibers. Inhibition of LOX activity by beta-aminoproprionitrile treatment results in a significant improvement in several metabolic parameters and further reduces local adipose tissue inflammation. Collectively, our observations are consistent with a model in which adipose tissue hypoxia serves as an early upstream initiator for adipose tissue dysfunction by inducing a local state of fibrosis.
Figures





References
-
- Brahimi-Horn, M. C., J. Chiche, and J. Pouyssegur. 2007. Hypoxia and cancer. J. Mol. Med. 851301-1307. - PubMed
-
- Brakenhielm, E., R. Cao, B. Gao, B. Angelin, B. Cannon, P. Parini, and Y. Cao. 2004. Angiogenesis inhibitor, TNP-470, prevents diet-induced and genetic obesity in mice. Circ. Res. 941579-1588. - PubMed
-
- Cancello, R., C. Henegar, N. Viguerie, S. Taleb, C. Poitou, C. Rouault, M. Coupaye, V. Pelloux, D. Hugol, J. L. Bouillot, A. Bouloumie, G. Barbatelli, S. Cinti, P. A. Svensson, G. S. Barsh, J. D. Zucker, A. Basdevant, D. Langin, and K. Clement. 2005. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 542277-2286. - PubMed
-
- Cho, C. H., Y. J. Koh, J. Han, H. K. Sung, H. Jong Lee, T. Morisada, R. A. Schwendener, R. A. Brekken, G. Kang, Y. Oike, T. S. Choi, T. Suda, O. J. Yoo, and G. Y. Koh. 2007. Angiogenic role of LYVE-1-positive macrophages in adipose tissue. Circ. Res. 100e47-e57. - PubMed
-
- Chun, T. H., K. B. Hotary, F. Sabeh, A. R. Saltiel, E. D. Allen, and S. J. Weiss. 2006. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell 125577-591. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
