In vitro and in vivo properties of dihydrophthalazine antifolates, a novel family of antibacterial drugs
- PMID: 19546364
- PMCID: PMC2737855
- DOI: 10.1128/AAC.00377-09
In vitro and in vivo properties of dihydrophthalazine antifolates, a novel family of antibacterial drugs
Abstract
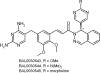
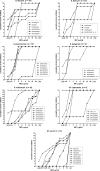
Racemic 2,4-diaminopyrimidine dihydrophthalazine derivatives BAL0030543, BAL0030544, and BAL0030545 exhibited low in vitro MICs toward small, selected panels of Enterococcus faecalis, Enterococcus faecium, Streptococcus pneumoniae, Moraxella catarrhalis, and Mycobacterium avium, though the compounds were less active against Haemophilus influenzae. The constellation of dihydrofolate reductases (DHFRs) present in 20 enterococci and 40 staphylococci was analyzed and correlated with the antibacterial activities of the dihydrophthalazines and trimethoprim. DHFRs encoded by dfrB, dfrA (S1 isozyme), dfrE, and folA were susceptible to the dihydrophthalazines, whereas DHFRs encoded by dfrG (S3 isozyme) and dfrF were not. Studies with the separated enantiomers of BAL0030543, BAL0030544, and BAL0030545 revealed preferential inhibition of susceptible DHFRs by the (R)-enantiomers. BAL0030543, BAL0030544, and BAL0030545 were well tolerated by mice during 5- and 10-day oral toxicity studies at doses of up to 400 mg/kg of body weight. Using a nonoptimized formulation, the dihydrophthalazines displayed acceptable oral bioavailabilities in mice, and efficacy studies with a septicemia model of mice infected with trimethoprim-resistant, methicillin-resistant Staphylococcus aureus gave 50% effective dose values in the range of 1.6 to 6.25 mg/kg.
Figures
References
-
- Barrow, E. W., W. J. Suling, L. E. Seitz, R. C. Reynolds, and W. W. Barrow. 2006. New antifolate inhibitors for Mycobacterium avium. Med. Chem. 2:505-510. - PubMed
-
- Blasi, F., D. J. Farrell, and L. Dubreuil. 2009. Antibacterial activity of telithromycin and comparators against pathogens isolated from patients with community-acquired respiratory tract infections: the Prospective Resistant Organism Tracking and Epidemiology for the Ketolide Telithromycin Study year 5 (2003-2004). Diagn. Microbiol. Infect. Dis. 63:302-308. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases