Protective importance of the myogenic response in the renal circulation
- PMID: 19546375
- PMCID: PMC2777749
- DOI: 10.1161/HYPERTENSIONAHA.109.133777
Protective importance of the myogenic response in the renal circulation
Conflict of interest statement
Figures




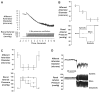
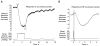

References
-
- US Renal Data System: USRDS 2005 Annual Data Report, Atlas of End Stage Renal Disease in the United States. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health; 2005.
-
- Bidani AK, Griffin KA. Long-term renal consequences of hypertension for normal and diseased kidneys. Curr Opin Nephrol Hypertens. 2002;11:73–80. - PubMed
-
- Bidani AK, Griffin KA. Pathophysiology of hypertensive renal damage: implications for therapy. Hypertens. 2004;44:1–7. - PubMed
-
- Griffin KA, Bidani AK. Progression of renal disease: the renoprotective specificity of renin angiotensin system blockade. Clin J Am Soc Nephrol. 2006;1:1054–1065. invited review. - PubMed
-
- Olson JL. Renal Disease caused by hypertension. In: Jennette JC, Olson JL, Schwartz MM, Silva FG, editors. Heptinstall's Pathology of the Kidney. sixth. II. Lippincott Williams & Wilkins; Philadelphia, PA: 2006. pp. 937–990.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

