Bovine lactadherin as a calcium-independent imaging agent of phosphatidylserine expressed on the surface of apoptotic HeLa cells
- PMID: 19546474
- PMCID: PMC2746724
- DOI: 10.1369/jhc.2009.953729
Bovine lactadherin as a calcium-independent imaging agent of phosphatidylserine expressed on the surface of apoptotic HeLa cells
Abstract
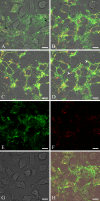
Bovine lactadherin holds a stereo-specific affinity for phosphatidylserine (PS) membrane domains and binds at PS concentrations lower than the benchmark PS probe, annexin V. Accordingly, lactadherin has recognized PS exposure on preapoptotic immortalized leukemia cells at an earlier time point than has annexin V. In the present study, the cervical cancer cell line HeLa has been employed as a model system to compare the topographic distribution of PS with the two PS binding proteins as adherent cells enter the apoptotic program. HeLa cells were cultured on glass-bottom Petri dishes, and apoptosis was induced by staurosporine. Fluorescence-labeled lactadherin and/or annexin V were used to detect PS exposure by confocal microscopy. Both lactadherin and annexin V staining revealed PS localized to plasma membrane rim and blebs. In addition, lactadherin identified PS exposure on long filopodia-like extensions, whereas annexin V internalized in granule-like structures. All in all, the data further delineate the differences in PS binding patterns of lactadherin and annexin V.
Figures


Similar articles
-
Measurement of annexin V uptake and lactadherin labeling for the quantification of apoptosis in adherent Tca8113 and ACC-2 cells.Braz J Med Biol Res. 2008 Sep;41(9):750-7. doi: 10.1590/s0100-879x2008000900002. Braz J Med Biol Res. 2008. PMID: 18820763
-
Lactadherin detects early phosphatidylserine exposure on immortalized leukemia cells undergoing programmed cell death.Cytometry A. 2006 Dec 1;69(12):1193-201. doi: 10.1002/cyto.a.20345. Cytometry A. 2006. PMID: 17123296
-
Lactadherin binding and phosphatidylserine expression on cell surface-comparison with annexin A5.Transl Res. 2006 Jul;148(1):19-25. doi: 10.1016/j.lab.2006.03.006. Transl Res. 2006. PMID: 16887494
-
Lactadherin: An unappreciated haemostasis regulator and potential therapeutic agent.Vascul Pharmacol. 2018 Feb;101:21-28. doi: 10.1016/j.vph.2017.11.006. Epub 2017 Nov 21. Vascul Pharmacol. 2018. PMID: 29169950 Review.
-
Annexin V, the regulator of phosphatidylserine-catalyzed inflammation and coagulation during apoptosis.Cell Mol Life Sci. 1997 Jun;53(6):527-32. doi: 10.1007/s000180050067. Cell Mol Life Sci. 1997. PMID: 9230931 Free PMC article. Review.
Cited by
-
Lactadherin's Multistate Binding Predicts Stable Membrane-Bound Conformations of Factors V and VIII's C Domains.Biochemistry. 2023 Oct 17;62(20):3020-3032. doi: 10.1021/acs.biochem.3c00274. Epub 2023 Sep 25. Biochemistry. 2023. PMID: 37747791 Free PMC article.
-
Membrane curvature and PS localize coagulation proteins to filopodia and retraction fibers of endothelial cells.Blood Adv. 2023 Jan 10;7(1):60-72. doi: 10.1182/bloodadvances.2021006870. Blood Adv. 2023. PMID: 35849711 Free PMC article.
-
Myosin-X is essential to the intercellular spread of HIV-1 Nef through tunneling nanotubes.J Cell Commun Signal. 2019 Jun;13(2):209-224. doi: 10.1007/s12079-018-0493-z. Epub 2018 Nov 15. J Cell Commun Signal. 2019. PMID: 30443895 Free PMC article.
-
Lactadherin orthologs inhibit migration of human, porcine and murine intestinal epithelial cells.Food Sci Nutr. 2017 May 15;5(4):934-942. doi: 10.1002/fsn3.479. eCollection 2017 Jul. Food Sci Nutr. 2017. PMID: 28748083 Free PMC article.
-
In vivo targeting of cell death using a synthetic fluorescent molecular probe.Apoptosis. 2011 Jul;16(7):722-31. doi: 10.1007/s10495-011-0601-5. Apoptosis. 2011. PMID: 21499791 Free PMC article.
References
-
- Albanyan A, Murphy MF, Rasmussen JT, Heegaard CW, Harrison P (2009) Measurement of phosphatidylserine exposure during storage of platelet concentrates using the novel probe lactadherin: a comparison study with annexin V. Transfusion 39:99–107 - PubMed
-
- Andersen MH, Berglund L, Rasmussen JT, Petersen TE (1997) Bovine PAS-6/7 binds αVβ5 integrin and anionic phospholipids through two domains. Biochemistry 36:5441–5446 - PubMed
-
- Andersen MH, Graversen H, Fedosov SN, Petersen TE, Rasmussen JT (2000) Functional analyses of two cellular binding domains of bovine lactadherin. Biochemistry 39:6200–6206 - PubMed
-
- Andree H, Reutelingsperger C, Hauptmann R, Hemker H, Hermens W, Willems G (1990) Binding of vascular anticoagulant α (VACa) to planar phospholipid bilayers. J Biol Chem 265:4923–4928 - PubMed
-
- Andree HA, Stuart MC, Hermens WT, Reutelingsperger CP, Hemker HC, Frederik PM, Willems GM (1992) Clustering of lipid-bound annexin V may explain its anticoagulant effect. J Biol Chem 267:17907–17912 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

