Direct crosstalk between mast cell-TNF and TNFR1-expressing endothelia mediates local tissue inflammation
- PMID: 19546478
- PMCID: PMC2731644
- DOI: 10.1182/blood-2008-11-187682
Direct crosstalk between mast cell-TNF and TNFR1-expressing endothelia mediates local tissue inflammation
Abstract
Signaling through tumor necrosis factor receptor 1 (TNFR1) controls bacterial infections and the induction of inflammatory Th1 cell-mediated autoimmune diseases. By dissecting Th1 cell-mediated delayed-type hypersensitivity responses (DTHRs) into single steps, we localized a central defect to the missing TNFR1 expression by endothelial cells (ECs). Adoptive transfer and mast cell knockin experiments into Kit(W)/Kit(W-v), TNF(-/-), and TNFR1(-/-) mice showed that the signaling defect exclusively affects mast cell-EC interactions but not T cells or antigen-presenting cells. As a consequence, TNFR1(-/-) mice had strongly reduced mRNA and protein expression of P-selectin, E-selectin, ICAM-1, and VCAM-1 during DTHR elicitation. In consequence, intravital fluorescence microscopy revealed up to 80% reduction of leukocyte rolling and firm adhesion in TNFR1(-/-) mice. As substitution of TNF(-/-) mice with TNF-producing mast cells fully restored DTHR in these mice, signaling of mast cell-derived TNF through TNFR1-expressing ECs is essential for the recruitment of leukocytes into sites of inflammation.
Figures

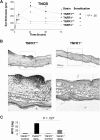
 ) and TNFR1+/+ (■) mice were challenged with TNCB. Ear thickness was measured before and at the indicated time points after TNCB challenge. Differences in ear thickness between TNFR1−/− (
) and TNFR1+/+ (■) mice were challenged with TNCB. Ear thickness was measured before and at the indicated time points after TNCB challenge. Differences in ear thickness between TNFR1−/− ( ) and TNFR1+/+ (■) were significant (P < .05) 24, 48, and 72 hours after ear challenge (24 hours: n = 16-27; 48 hours and 72 hours: n = 6 or 7). (B) Reduced PMN infiltrates, tissue necrosis, and edema in ear tissue from TNFR1−/− mice 24 hours after TNCB challenge. Hematoxylin and eosin–stained ear sections from TNFR1+/+ (left: top represents overview; bottom represents detail) and TNFR1−/− mice (right: top represents overview; bottom represents detail; n = 13-15). (C) PMN recruitment is TNFR1-dependent. MPO activity in protein extracts from ear tissue from TNFR1−/− and TNFR1+/+ mice (n = 3).
) and TNFR1+/+ (■) were significant (P < .05) 24, 48, and 72 hours after ear challenge (24 hours: n = 16-27; 48 hours and 72 hours: n = 6 or 7). (B) Reduced PMN infiltrates, tissue necrosis, and edema in ear tissue from TNFR1−/− mice 24 hours after TNCB challenge. Hematoxylin and eosin–stained ear sections from TNFR1+/+ (left: top represents overview; bottom represents detail) and TNFR1−/− mice (right: top represents overview; bottom represents detail; n = 13-15). (C) PMN recruitment is TNFR1-dependent. MPO activity in protein extracts from ear tissue from TNFR1−/− and TNFR1+/+ mice (n = 3).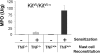
 ) or TNF+/+ (■) mast cells. Ear tissue was harvested 24 hours after TNCB challenge from sensitized and naive KitW/KitW-v mice (n = 2).
) or TNF+/+ (■) mast cells. Ear tissue was harvested 24 hours after TNCB challenge from sensitized and naive KitW/KitW-v mice (n = 2).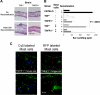
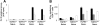
 ) CD4+ T cells from either naive TNFR1+/+ or TNFR1−/− mice, or primed TNFR1+/+ or TNFR1−/− mice were stimulated for 72 hours with 5 × 105 of either unmodified (
) CD4+ T cells from either naive TNFR1+/+ or TNFR1−/− mice, or primed TNFR1+/+ or TNFR1−/− mice were stimulated for 72 hours with 5 × 105 of either unmodified ( and □) or TNCB-modified (■ and
and □) or TNCB-modified (■ and  ) APCs; [3H] thymidine was added for the final 6 hours. (B) Frequency of IFN-γ–producing TNCB-specific CD4+ T cells, 5 × 105 (■), or 2.5 × 105 (
) APCs; [3H] thymidine was added for the final 6 hours. (B) Frequency of IFN-γ–producing TNCB-specific CD4+ T cells, 5 × 105 (■), or 2.5 × 105 ( ) T cells from either naive or sensitized TNFR1+/+ or TNFR1−/− mice were stimulated on anti–IFN-γ mAb-coated 96-well plates with 5 × 105 either unmodified APCs (
) T cells from either naive or sensitized TNFR1+/+ or TNFR1−/− mice were stimulated on anti–IFN-γ mAb-coated 96-well plates with 5 × 105 either unmodified APCs ( , third column) or TNBS-modified APCs (■ and
, third column) or TNBS-modified APCs (■ and  ). After 48 hours of incubation, ELISPOT assay was developed. Control T cells were stimulated with 10 μg/mL concanavalin A and APCs (□); 3 independent experiments.
). After 48 hours of incubation, ELISPOT assay was developed. Control T cells were stimulated with 10 μg/mL concanavalin A and APCs (□); 3 independent experiments.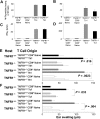
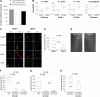
 ) TNCB-specific T cells (105) of the indicated origin were stimulated for 24 hours with 5 × 105 hapten-modified APCs. [3H] thymidine was added for the final 6 hours. (C-D) TNFR1+/+ (■) or TNFR1−/− (
) TNCB-specific T cells (105) of the indicated origin were stimulated for 24 hours with 5 × 105 hapten-modified APCs. [3H] thymidine was added for the final 6 hours. (C-D) TNFR1+/+ (■) or TNFR1−/− ( ) T cells (105) from the indicated origin were stimulated for 24 hours with 5.0 × 105 hapten-modified APCs. Supernatants were harvested after 24 hours, and the IFN-γ content was determined by enzyme-linked immunosorbent assay. (E-F) Efficient DTHRs require TNFR1-expressing resident cells. Th1 or Tc1 cell lines that were either TNFR1−/− or TNFR1+/+ were transferred intracutaneously into ears of naive TNFR1−/− or TNFR1+/+ mice, 0.5 hours before challenge with TNCB (n = 3-7). Ear swelling was determined 24 hours later.
) T cells (105) from the indicated origin were stimulated for 24 hours with 5.0 × 105 hapten-modified APCs. Supernatants were harvested after 24 hours, and the IFN-γ content was determined by enzyme-linked immunosorbent assay. (E-F) Efficient DTHRs require TNFR1-expressing resident cells. Th1 or Tc1 cell lines that were either TNFR1−/− or TNFR1+/+ were transferred intracutaneously into ears of naive TNFR1−/− or TNFR1+/+ mice, 0.5 hours before challenge with TNCB (n = 3-7). Ear swelling was determined 24 hours later.References
-
- Müller-Hermelink N, Braumuller H, Pichler B, et al. TNFR1 signaling and IFN-gamma signaling determine whether T cells induce tumor dormancy or promote multistage carcinogenesis. Cancer Cell. 2008;13:507–518. - PubMed
-
- Feldmann M, Steinman L. Design of effective immunotherapy for human autoimmunity. Nature. 2005;435:612–619. - PubMed
-
- Sayed BA, Christy A, Quirion MR, Brown MA. The master switch: the role of mast cells in autoimmunity and tolerance. Annu Rev Immunol. 2008;26:705–739. - PubMed
-
- Echtenacher B, Mannel DN, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

