Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice
- PMID: 19546507
- PMCID: PMC2701881
- DOI: 10.1172/JCI38714
Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice
Abstract
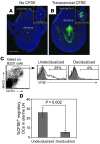
Embryo implantation induces formation of the decidua, a stromal cell-derived structure that encases the fetus and placenta. Using the mouse as a model organism, we have found that this tissue reaction prevents DCs stationed at the maternal/fetal interface from migrating to the lymphatic vessels of the uterus and thus reaching the draining lymph nodes. Strikingly, decidual DCs remained immobile even after being stimulated with LPS and exhibiting responsiveness to CCL21, the chemokine that drives DC entry into lymphatic vessels. An analysis of maternal T cell reactivity toward a surrogate fetal/placental antigen furthermore revealed that regional T cell responses toward the fetus and placenta were driven by passive antigen transport and thus the tolerogenic mode of antigen presentation that predominates when there is negligible input from tissue-resident DCs. Indeed, the lack of involvement of tissue-resident DCs in the T cell response to the fetal allograft starkly contrasts with their prominent role in organ transplant rejection. Our results suggest that DC entrapment within the decidua minimizes immunogenic T cell exposure to fetal/placental antigens and raise the possibility that impaired development or function of the human decidua, which unlike that of the mouse contains lymphatic vessels, might lead to pathological T cell activation during pregnancy.
Figures








Comment in
-
Restraining order for dendritic cells: all quiet on the fetal front.J Clin Invest. 2009 Jul;119(7):1854-7. doi: 10.1172/jci39946. J Clin Invest. 2009. PMID: 19603541 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

