The shock of aging: molecular chaperones and the heat shock response in longevity and aging--a mini-review
- PMID: 19546513
- PMCID: PMC2754743
- DOI: 10.1159/000225957
The shock of aging: molecular chaperones and the heat shock response in longevity and aging--a mini-review
Abstract
Background: Aging can be thought of as the collision between destructive processes that act on cells and organs over the lifetime and the responses that promote homeostasis, vitality and longevity. However, the precise mechanisms that determine the rates of aging in organisms are not known.
Objective: Macromolecules such as proteins are continuously exposed to potential damaging agents that can cause loss of molecular function and depletion of cell populations over the lifetime of essential organs. One of the key homeostatic responses involved in maintaining longevity is the induction of heat shock proteins (HSPs), a conserved reaction to damaged intracellular proteins. We aim to discuss how the interplay between protein damage and its repair or removal from the cell may influence longevity and aging.
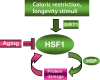
Methods: We have reviewed experiments carried out in mammalian and non-mammalian organisms on molecular chaperones and the transcription factor (heat shock factor 1, HSF1) responsible for their expression. We have discussed mechanisms through which these molecules are regulated in cells, respond to stimuli that enhance longevity and become impaired during aging.
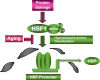
Results: The transcription factor HSF1 initiates the prolific induction of HSP when cells are exposed to protein damage. HSPs are molecular chaperones that protect the proteome by folding denatured polypeptides and promoting the degradation of severely damaged proteins. Activation of HSF1 is coupled functionally to fundamental pathways of longevity and orchestrates the evasion of aging through HSP induction and antagonism of protein aggregation. In addition to mediating protein quality control, some HSPs such as Hsp27 and Hsp70 directly protect cells against damage-induced entry into death pathways. However, the heat shock response declines in potency over the lifetime, and enfeeblement of the response contributes to aging by permitting the emergence of protein aggregation diseases, reduction in cellular vigor and decreased longevity.
Conclusions: Molecular chaperones play an important role in the deterrence of protein damage during aging and their expression is required for longevity. Chemical stimulation of HSP synthesis might therefore be a significant strategy in future design of antiaging pharmaceuticals.
Copyright 2009 S. Karger AG, Basel.
Figures

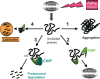
References
-
- Westphal CH, Dipp MA, Guarente L. A therapeutic role for sirtuins in diseases of aging? Trends Biochem Sci. 2007;32:555–560. - PubMed
-
- Lindquist S, Craig EA. The heat shock proteins. Annu Rev Genet. 1988;22:631–637. - PubMed
-
- Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006;5:2592–2601. - PubMed
-
- Ellis RJ. Protein misassembly: macromolecular crowding and molecular chaperones. Adv Exp Med Biol. 2007;594:1–13. - PubMed
-
- Sherman MY, Goldberg AL. Cellular defenses against unfolded proteins: a cell biologist thinks about neurodegenerative diseases. Neuron. 2001;29:15–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

