Maraviroc concentrates in the cervicovaginal fluid and vaginal tissue of HIV-negative women
- PMID: 19546811
- PMCID: PMC2751766
- DOI: 10.1097/QAI.0b013e3181ae69c5
Maraviroc concentrates in the cervicovaginal fluid and vaginal tissue of HIV-negative women
Abstract
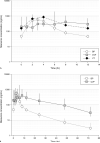
Objective: To compare single- and multiple-dose maraviroc exposures in cervicovaginal fluid (CVF) and vaginal tissue (VT) with blood plasma (BP) and quantify maraviroc protein binding in CVF.
Design: Open-label pharmacokinetic study.
Methods: In 12 HIV-negative women, 7 paired CVF and BP samples were collected over 12 hours after 1 maraviroc dose. Subjects then received maraviroc twice daily for 7 days. After the last dose, subjects underwent CVF and BP sampling as on day 1, with additional sampling during terminal elimination. VT biopsies were obtained at steady state.
Results: Day 1 and day 7 median maraviroc CVF AUCtau were 1.9- and 2.7-fold higher, respectively, than BP. On day 1, 6 of 12 subjects had detectable maraviroc CVF concentrations within 1 hour; 12 of 12 were detectable within 2 hours, and all exceeded the protein-free IC90. On day 7, maraviroc CVF protein binding was 7.6% and the VT AUCtau was 1.9-fold higher than BP. Maraviroc CVF concentrations 72 hours after dose and BP concentrations 12 hours after dose were similar.
Conclusions: Higher maraviroc exposure in the female genital tract provides a pharmacologic basis for further evaluation of chemokine receptor 5 antagonists in HIV infection prophylaxis. This is the first study to report antiretroviral VT concentrations, CVF protein binding, and CVF terminal elimination.
Figures

Similar articles
-
Single and multiple dose pharmacokinetics of dolutegravir in the genital tract of HIV-negative women.Antivir Ther. 2013;18(8):1005-13. doi: 10.3851/IMP2665. Epub 2013 Jul 31. Antivir Ther. 2013. PMID: 23899439 Free PMC article. Clinical Trial.
-
Cervicovaginal and Rectal Fluid as a Surrogate Marker of Antiretroviral Tissue Concentration: Implications for Clinical Trial Design.J Acquir Immune Defic Syndr. 2016 Aug 15;72(5):498-506. doi: 10.1097/QAI.0000000000000996. J Acquir Immune Defic Syndr. 2016. PMID: 26999532 Free PMC article.
-
Phase 1 Safety, Pharmacokinetics, and Pharmacodynamics of Dapivirine and Maraviroc Vaginal Rings: A Double-Blind Randomized Trial.J Acquir Immune Defic Syndr. 2015 Nov 1;70(3):242-9. doi: 10.1097/QAI.0000000000000702. J Acquir Immune Defic Syndr. 2015. PMID: 26034880 Free PMC article. Clinical Trial.
-
Maraviroc: pharmacokinetics and drug interactions.Antivir Ther. 2009;14(5):607-18. Antivir Ther. 2009. PMID: 19704163 Review.
-
Maraviroc: a CCR5-receptor antagonist for the treatment of HIV-1 infection.Clin Ther. 2008 Jul;30(7):1228-50. doi: 10.1016/s0149-2918(08)80048-3. Clin Ther. 2008. PMID: 18691983 Review.
Cited by
-
Differential penetration of raltegravir throughout gastrointestinal tissue: implications for eradication and cure.AIDS. 2013 Jun 1;27(9):1413-9. doi: 10.1097/QAD.0b013e32835f2b49. AIDS. 2013. PMID: 23945503 Free PMC article.
-
Darunavir, ritonavir, and etravirine pharmacokinetics in the cervicovaginal fluid and blood plasma of HIV-infected women.Antimicrob Agents Chemother. 2011 Mar;55(3):1120-2. doi: 10.1128/AAC.00889-10. Epub 2010 Dec 20. Antimicrob Agents Chemother. 2011. PMID: 21173188 Free PMC article. Clinical Trial.
-
Maraviroc: a review of its use in HIV infection and beyond.Drug Des Devel Ther. 2015 Oct 1;9:5447-68. doi: 10.2147/DDDT.S90580. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26491256 Free PMC article. Review.
-
Promising prevention approaches: tenofovir gel and prophylactic use of antiretroviral medications.Curr HIV/AIDS Rep. 2011 Dec;8(4):241-8. doi: 10.1007/s11904-011-0094-4. Curr HIV/AIDS Rep. 2011. PMID: 22002729 Free PMC article. Review.
-
Pre-exposure prophylaxis for HIV prevention: how to predict success.Lancet. 2012 Jun 30;379(9835):2409-2411. doi: 10.1016/S0140-6736(11)61852-7. Epub 2011 Dec 6. Lancet. 2012. PMID: 22153566 Free PMC article. No abstract available.
References
-
- UNAIDS/WHO AIDS epidemic update. AIDS Epidemic Update. 2007. [December 1, 2007]. Available online at: http://data.unaids.org/pub/EPISlides/2007/2007_epiupdate_en.pdf.
-
- Mocroft A, Ledergerber B, Katlama C, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362:22–29. - PubMed
-
- Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. - PubMed
-
- National Institutes of Health: National Institute of Allergy and Infectious Disease . HIV infection in women. In: Anderson JR, editor. AIDSInfo. Department of Health and Human Services; Rockville: 2006.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

