Slow progressive conduction and contraction defects in loss of Nkx2-5 mice after cardiomyocyte terminal differentiation
- PMID: 19546853
- PMCID: PMC2733927
- DOI: 10.1038/labinvest.2009.59
Slow progressive conduction and contraction defects in loss of Nkx2-5 mice after cardiomyocyte terminal differentiation
Abstract
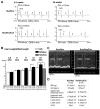
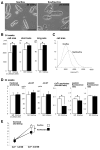
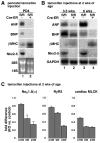
Mutations in homeoprotein NKX2-5 are linked to human congenital heart disease, resulting in various cardiac anomalies, as well as in postnatal progressive conduction defects and occasional left ventricular dysfunction; yet the function of Nkx2-5 in the postnatal period is largely unexplored. In the heart, the majority of cardiomyocytes are believed to complete cell-cycle withdrawal shortly after birth, which is generally accompanied by a re-organization of chromatin structure shown in other tissues. We reasoned that the effects of the loss of Nkx2-5 in mice may be different after cell-cycle withdrawal compared with those of the perinatal loss of Nkx2-5, which results in rapid conduction and contraction defects within 4 days after the deletion of Nkx2-5 alleles (Circ Res. 2008;103:580). In this study, floxed-Nkx2-5 alleles were deleted using tamoxifen-inducible Cre transgene (Cre-ER) beginning at 2 weeks of age. The loss of Nkx2-5 beginning at 2 weeks of age resulted in conduction and contraction defects similar to the perinatal loss of Nkx2-5, however, with a substantially slower disease progression shown by 1 degrees atrioventricular block at 6 weeks of age (4 weeks after tamoxifen injections) and heart enlargement after 12 weeks of age (10 weeks after tamoxifen injections). The phenotypes were accompanied by a slower and smaller degree of reduction of several critical Nkx2-5 downstream targets that were observed in mice with a perinatal loss of Nkx2-5. These results suggest that Nkx2-5 is necessary for proper conduction and contraction after 2 weeks of age, but with a substantially distinct level of necessity at 2 weeks of age compared with that in the perinatal period.
Conflict of interest statement
None declared.
Figures


References
-
- Harvey RP. NK-2 homeobox genes and heart development. Dev Biol. 1996;178(2):203–216. - PubMed
-
- Harvey RP, Rosenthal N. Heart Development. Academic Press; 1999.
-
- Lints TJ, Parsons LM, Hartley L, Lyons I, Harvey RP. Nkx-2.5: a novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. Development. 1993;119(2):419–431. - PubMed
-
- Kasahara H, Bartunkova S, Schinke M, Tanaka M, Izumo S. Cardiac and extracardiac expression of Csx/Nkx2.5 homeodomain protein. Circ Res. 1998;82(9):936–946. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

