Osteopetrosis with micro-lacunar resorption because of defective integrin organization
- PMID: 19546854
- PMCID: PMC2856930
- DOI: 10.1038/labinvest.2009.58
Osteopetrosis with micro-lacunar resorption because of defective integrin organization
Abstract
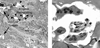
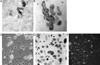
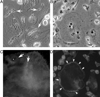
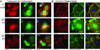
In vitro differentiated monocytes were used to characterize the cellular defect in a type of osteopetrosis with minimally functional osteoclasts, in which defects associated with common causes of osteopetrosis were excluded by gene sequencing. Monocytes from the blood of a 28-year-old patient were differentiated in media with RANKL and CSF-1. Cell fusion, acid compartments within cells, and tartrate resistant acid phosphatase (TRAP) activity were normal. However, the osteoclasts made abnormally small pits on the dentine. Phalloidin labeling showed that the cell attachments lacked the peripheral ring structure that supports lacunar resorption. Instead, the osteoclasts had clusters of podosomes near the center of cell attachments. Antibody to the alphavbeta3 integrin pair or to the C-terminal of beta3 did not label podosomes, but antibody to alphav labeled them. Western blots using antibody to the N-terminal of beta3 showed a protein of reduced size. Integrins beta1 and beta5 were upregulated, but, in contrast to observations in beta3 defects, alpha2 had not increased. The rho-GTP exchange protein Vav3, a key attachment organizing protein, did not localize normally with peripheral attachment structures. Vav3 forms of 70 kD and 90 kD were identified on western blots. However, the proteins beta3 integrin, Vav3, Plekhm1, and Src, implicated in attachment defects, had normal exon sequences. In this new type of osteopetrosis, the integrin-organizing complex is dysfunctional, and at least two attachment proteins may be partially degraded.
Figures


References
-
- Tolar J, Teitelbaum SL, Orchard PJ. Osteopetrosis. N Engl J Med. 2004;351:2839–2349. - PubMed
-
- Blair HC, Teitelbaum SL, Ghiselli R, et al. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science. 1989;245:855–857. - PubMed
-
- Li YP, Chen W, Liang Y, et al. Atp6i-deficient mice exhibit severe osteopetrosis due to loss of osteoclast-mediated extracellular acidification. Nat Genet. 1999;23:447–451. - PubMed
-
- Blair HC, Borysenko CW, Villa A, et al. In vitro differentiation of CD14 cells from osteopetrotic subjects: contrasting phenotypes with TCIRG1, CLCN7, and attachment defects. J Bone Miner Res. 2004;19:1329–1338. - PubMed
-
- Scheel O, Zdebik AA, Lourdel S, et al. Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature. 2005;436:424–427. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

