Androgens induce oxidative stress and radiation resistance in prostate cancer cells though NADPH oxidase
- PMID: 19546883
- PMCID: PMC2834342
- DOI: 10.1038/pcan.2009.24
Androgens induce oxidative stress and radiation resistance in prostate cancer cells though NADPH oxidase
Abstract
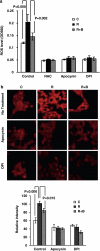
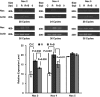
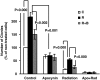
Androgen deprivation therapy (ADT) facilitates the response of prostate cancer (PC) to radiation. Androgens have been shown to induce elevated basal levels of reactive oxygen species (ROS) in PC, leading to adaptation to radiation-induced cytotoxic oxidative stress. Here, we show that androgens increase the expression of p22(phox) and gp91(phox) subunits of NADPH oxidase (NOX) and ROS production by NOX2 and NOX4 in PC. Pre-radiation treatment of 22Rv1 human PC cells with NOX inhibitors sensitize the cells to radiation similarly to ADT, suggesting that their future usage may spare the need for adjuvant ADT in PC patients undergoing radiation.
Figures



Similar articles
-
Inter-related in vitro effects of androgens, fatty acids and oxidative stress in prostate cancer: a mechanistic model supporting prevention strategies.Int J Oncol. 2010 Oct;37(4):761-6. doi: 10.3892/ijo_00000725. Int J Oncol. 2010. PMID: 20811696
-
Early NADPH oxidase-2 activation is crucial in phenylephrine-induced hypertrophy of H9c2 cells.Cell Signal. 2014 Sep;26(9):1818-24. doi: 10.1016/j.cellsig.2014.04.018. Epub 2014 May 2. Cell Signal. 2014. PMID: 24794531 Free PMC article.
-
NOX2, p22phox and p47phox are targeted to the nuclear pore complex in ischemic cardiomyocytes colocalizing with local reactive oxygen species.Cell Physiol Biochem. 2011;27(5):471-8. doi: 10.1159/000329968. Epub 2011 Jun 15. Cell Physiol Biochem. 2011. PMID: 21691064
-
NADPH oxidases as novel pharmacologic targets against influenza A virus infection.Mol Pharmacol. 2014 Dec;86(6):747-59. doi: 10.1124/mol.114.095216. Epub 2014 Oct 9. Mol Pharmacol. 2014. PMID: 25301784 Review.
-
The defense and signaling role of NADPH oxidases in eukaryotic cells : Review.Wien Med Wochenschr. 2018 Sep;168(11-12):286-299. doi: 10.1007/s10354-018-0640-4. Epub 2018 Aug 6. Wien Med Wochenschr. 2018. PMID: 30084091 Free PMC article. Review.
Cited by
-
A Mini-Review of Reactive Oxygen Species in Urological Cancer: Correlation with NADPH Oxidases, Angiogenesis, and Apoptosis.Int J Mol Sci. 2017 Oct 22;18(10):2214. doi: 10.3390/ijms18102214. Int J Mol Sci. 2017. PMID: 29065504 Free PMC article. Review.
-
The Association between NADPH Oxidase 2 (NOX2) and Drug Resistance in Cancer.Curr Cancer Drug Targets. 2024;24(12):1195-1212. doi: 10.2174/0115680096277328240110062433. Curr Cancer Drug Targets. 2024. PMID: 38362697 Review.
-
Efficacy characteristics of different therapeutic modalities for locally advanced prostate cancer: a Bayesian network meta-analysis of randomized controlled trials.Ann Transl Med. 2018 Sep;6(18):358. doi: 10.21037/atm.2018.08.38. Ann Transl Med. 2018. PMID: 30370285 Free PMC article.
-
Nrf1 and Nrf2 transcription factors regulate androgen receptor transactivation in prostate cancer cells.PLoS One. 2014 Jan 22;9(1):e87204. doi: 10.1371/journal.pone.0087204. eCollection 2014. PLoS One. 2014. PMID: 24466341 Free PMC article.
-
Critical role of antioxidant programs in enzalutamide-resistant prostate cancer.Oncogene. 2023 Jul;42(30):2347-2359. doi: 10.1038/s41388-023-02756-w. Epub 2023 Jun 24. Oncogene. 2023. PMID: 37355762 Free PMC article.
References
-
- Nichol A, Chung P, Lockwood G, Rosewall T, Divanbiegi L, Sweet J, et al. A phase II study of localized prostate cancer treated to 75.6 Gy with 3D conformal radiotherapy. Radiother Oncol. 2005;76:11–17. - PubMed
-
- Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff RO, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360:103–106. - PubMed
-
- D'Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA. 2004;292:821–827. - PubMed
-
- Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–244. - PubMed
-
- Woodward WA, Wachsberger P, Burd R, Dicker AP. Effects of androgen suppression and radiation on prostate cancer suggest a role for angiogenesis blockade. Prostate Cancer Prostatic Dis. 2005;8:127–132. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

