Types of study in medical research: part 3 of a series on evaluation of scientific publications
- PMID: 19547627
- PMCID: PMC2689572
- DOI: 10.3238/arztebl.2009.0262
Types of study in medical research: part 3 of a series on evaluation of scientific publications
Abstract
Background: The choice of study type is an important aspect of the design of medical studies. The study design and consequent study type are major determinants of a study's scientific quality and clinical value.
Methods: This article describes the structured classification of studies into two types, primary and secondary, as well as a further subclassification of studies of primary type. This is done on the basis of a selective literature search concerning study types in medical research, in addition to the authors' own experience.
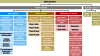
Results: Three main areas of medical research can be distinguished by study type: basic (experimental), clinical, and epidemiological research. Furthermore, clinical and epidemiological studies can be further subclassified as either interventional or noninterventional.
Conclusions: The study type that can best answer the particular research question at hand must be determined not only on a purely scientific basis, but also in view of the available financial resources, staffing, and practical feasibility (organization, medical prerequisites, number of patients, etc.).
Keywords: basic research; clinical research; epidemiology; literature search; study type.
Figures

References
-
- Bortz J, Döring N. Forschungsmethoden und Evaluation. Springer: Berlin, Heidelberg, New York; 2002. pp. 39–84.
-
- Bortz J, Döring N. Forschungsmethoden und Evaluation. Berlin, Heidelberg, New York: Springer; 2002. 37 pp.
-
- Fletcher RH, Fletcher SW. Klinische Epidemiologie. Grundlagen und Anwendung. Bern: Huber; 2007. pp. 1–327.
-
- Altman DG. Practical statistics for medical research. 1. Aufl. Boca Raton, London, New York, Washington D.C.: Chapman & Hall; 1991. pp. 1–499.
-
- Schumacher M, Schulgen G. Methodik klinischer Studien. 2. Aufl. Berlin, Heidelberg, New York: Springer; 2007. pp. 1–436.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

