Chlamydia pneumoniae hides inside apoptotic neutrophils to silently infect and propagate in macrophages
- PMID: 19547701
- PMCID: PMC2695784
- DOI: 10.1371/journal.pone.0006020
Chlamydia pneumoniae hides inside apoptotic neutrophils to silently infect and propagate in macrophages
Abstract
Background: Intracellular pathogens have developed elaborate strategies for silent infection of preferred host cells. Chlamydia pneumoniae is a common pathogen in acute infections of the respiratory tract (e.g. pneumonia) and associated with chronic lung sequelae in adults and children. Within the lung, alveolar macrophages and polymorph nuclear neutrophils (PMN) are the first line of defense against bacteria, but also preferred host phagocytes of chlamydiae.
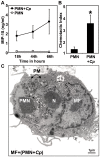
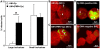
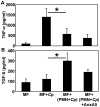
Methodology/principal findings: We could show that C. pneumoniae easily infect and hide inside neutrophil granulocytes until these cells become apoptotic and are subsequently taken up by macrophages. C. pneumoniae infection of macrophages via apoptotic PMN results in enhanced replicative activity of chlamydiae when compared to direct infection of macrophages, which results in persistence of the pathogen. Inhibition of the apoptotic recognition of C. pneumoniae infected PMN using PS- masking Annexin A5 significantly lowered the transmission of chlamydial infection to macrophages. Transfer of apoptotic C. pneumoniae infected PMN to macrophages resulted in an increased TGF-ss production, whereas direct infection of macrophages with chlamydiae was characterized by an enhanced TNF-alpha response.
Conclusions/significance: Taken together, our data suggest that C. pneumoniae uses neutrophil granulocytes to be silently taken up by long-lived macrophages, which allows for efficient propagation and immune protection within the human host.
Conflict of interest statement
Figures




References
-
- Gieffers J, van Zandbergen G, Rupp J, Sayk F, Kruger S, Ehlers S, Solbach W, Maass M. Phagocytes transmit Chlamydia pneumoniae from the lungs to the vasculature. Eur Respir J. 2004;23:506–510. - PubMed
-
- Yang ZP, Cummings PK, Patton DL, Kuo CC. Ultrastructural lung pathology of experimental Chlamydia pneumoniae pneumonitis in mice. J Infect Dis. 1994;170:464–467. - PubMed
-
- Jahn HU, Krull M, Wuppermann FN, Klucken AC, Rosseau S, Seybold J, Hegemann JH, Jantos CA, Suttorp N. Infection and activation of airway epithelial cells by Chlamydia pneumoniae. J Infect Dis. 2000;182:1678–1687. - PubMed
-
- van Zandbergen G, Gieffers J, Kothe H, Rupp J, Bollinger A, Aga E, Klinger M, Brade H, Dalhoff K, Maass M, Solbach W, Laskay T. Chlamydia pneumoniae multiply in neutrophil granulocytes and delay their spontaneous apoptosis. J Immunol. 2004;172:1768–1776. - PubMed
-
- Bartels C, Maass M, Bein G, Malisius R, Brill N, Bechtel JF, Sayk F, Feller AC, Sievers HH. Detection of Chlamydia pneumoniae but not cytomegalovirus in occluded saphenous vein coronary artery bypass grafts. Circulation. 1999;99:879–882. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

