C-H bond functionalization via hydride transfer: synthesis of dihydrobenzopyrans from ortho-vinylaryl akyl ethers
- PMID: 19548698
- PMCID: PMC2954889
- DOI: 10.1021/ol900915p
C-H bond functionalization via hydride transfer: synthesis of dihydrobenzopyrans from ortho-vinylaryl akyl ethers
Abstract
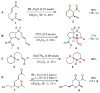
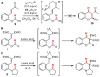
The hydride transfer initiated cyclization ("HT-cyclization") of aryl alkyl ethers, which leads to direct coupling of sp(3) C-H bonds and activated alkenes, is reported. Readily available salicylaldehyde derived ethers are converted in one step to dihydrobenzopyrans, an important class of heteroarenes frequently found in biologically active compounds. This process has not been previously reported, in contrast to known HT-cyclizations of the corresponding tert-amines ("tert-amino effect" reactions).
Figures
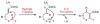
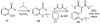
References
-
- Godula K, Sames D. Science. 2006;312:67–72. - PubMed
-
-
Some recent examples: Chatani N, Asaumi T, Yorimitsu S, Ikeda T, Kakiuchi F, Murai S. J Am Chem Soc. 2001;123:10935–10941.DeBoef B, Pastine SJ, Sames D. J Am Chem Soc. 2004;126:6556–6557.Shi L, Tu YQ, Wang M, Zhang FM, Fan CA, Zhao YM, Xia WJ. J Am Chem Soc. 2005;127:10836–10837.Kubiak R, Prochnow I, Doye S. Angew Chem Int Ed. 2009;48:1153–1156.Bexrud JA, Eisenberger P, Leitch DC, Payne PR, Schafer LL. J Am Chem Soc. 2009;131:2116–2118.
-
-
-
C-C bond formation at the α-position of ethers and carbamates can be achieved under neutral conditions via catalytic carbene insertion reactions. Davies HML, Venkataramani C, Hansen T, Hopper DW. J Am Chem Soc. 2003;125:6462–6468.Davies HML, Beckwith REJ, Antoulinakis EG, Jin Q. J Org Chem. 2003;68:6126–6132.
-
-
-
Pastine SJ, McQuaid KM, Sames D. J Am Chem Soc. 2005;127:12180–12181.Pastine SJ, Sames D. Org Lett. 2005;7:5429–5431.McQuaid KM, Sames D. J Am Chem Soc. 2009;131:402–403.For a commentary on this area of research, see: Tobisu M, Chatani N. Angew Chem Int Ed. 2006;45:1683–1684.For a recent example from other laboratories see: Shikanai D, Murase H, Hata T, Urabe H. J Am Chem Soc. 2009;131:3166–3167.
-
-
-
For recent examples of α-alkylation of ethers using an external oxidant, see : Tu W, Liu L, Floreancig PE. Angew Chem Int Ed. 2008;47:4184–4187.Li Z, Yu R, Li H. Angew Chem Int Ed. 2008;47:7497–7500.Cao K, Jiang YJ, Zhang SY, Fan CA, Tu YQ, Pan YJ. Tetrahedron Lett. 2008;49:4652–4654.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

