Patient-reported outcomes in a phase III, randomized study of sunitinib versus interferon-{alpha} as first-line systemic therapy for patients with metastatic renal cell carcinoma in a European population
- PMID: 19549706
- PMCID: PMC2768734
- DOI: 10.1093/annonc/mdp067
Patient-reported outcomes in a phase III, randomized study of sunitinib versus interferon-{alpha} as first-line systemic therapy for patients with metastatic renal cell carcinoma in a European population
Abstract
Background: The purpose of this study is to evaluate the impact on the health-related quality of life (HRQoL) of sunitinib versus interferon-alpha (IFN-alpha) treatment in patients with metastatic renal cell carcinoma (mRCC).
Patients and methods: In all, 304 mRCC patients (European cohort) were randomized 1 : 1 to receive sunitinib (50 mg/day for 4 weeks, followed by 2 weeks off) or IFN-alpha (9 million units s.c. injection three times/week). The following questionnaires were completed (days 1 and 28 per cycle): Functional Assessment of Cancer Therapy-General (FACT-G), the FACT-Kidney Symptom Index and the EuroQol Group's EQ-5D self-report questionnaire (EQ-5D). Results correspond to an ongoing trial with progression-free survival time as primary end point, and patients were still being followed up. Data were analyzed using repeated measures mixed effects models (MEMs) that allow the inclusion of initial differences and uncompleted repeated measures, with the assumption of data missing at random. Six-cycle results were included.
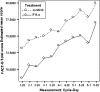
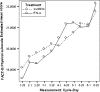
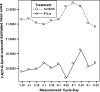
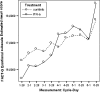
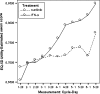
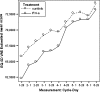
Results: Results consistently showed that patients in sunitinib group experienced statistically significantly milder kidney-related symptoms, better cancer-specific HRQoL and general health status (in social utility scores) during the study period as measured by these patient-reported outcome end points. No statistical differences between groups were found on the FACT-G physical well-being subscale or the EQ-5D VAS values.
Conclusions: Results from MEM showed the sunitinib's benefit on HRQoL compared with IFN-alpha.
Figures



References
-
- Food and Drug Administration (FDA) Guidance for Industry. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labelling Claims. http://www.fda.gov/cder/guidance/index.html. (13 March 2008, date last accessed) - PMC - PubMed
-
- Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124. - PubMed
-
- Koff RS. Impaired health-related quality of life in chronic hepatitis C: the how, but not the why. Hepatology. 1999;29(1):277–279. - PubMed
-
- Copley-Merriman K, Jackson J, Boyer JG, et al. Invited Paper D. Industry perspectives regarding outcomes research in oncology. In: Lipscomb J, Gotay CC, Snyder C, editors. Outcomes Assessment in Cancer. Cambridge, UK: Cambridge University Press; 2005.
-
- FACIT. Functional Assessment of Chronic Illness Therapy. http://www.facit.org/qview/qlist.aspx. (15 March 2008, date last accessed)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

