Formation of harmful algal blooms cannot be explained by allelopathic interactions
- PMID: 19549831
- PMCID: PMC2708709
- DOI: 10.1073/pnas.0900964106
Formation of harmful algal blooms cannot be explained by allelopathic interactions
Abstract
Many planktonic microalgae produce a range of toxins and may form harmful algal blooms. One hypothesis is that some toxins are allelopathic, suppressing the growth of competitors, and it has been suggested that allelopathy may be one important mechanism causing algal blooms. In a metaanalysis of recent experimental work, we looked for evidence that allelopathy may explain the initiation of algal blooms. With few exceptions, allelopathic effects were only significant at very high cell densities typical of blooms. We conclude that there is no experimental support for allelopathy at prebloom densities, throwing doubts on allelopathy as a mechanism in bloom formation. Most studies tested allelopathy using cell-free manipulations. With simple models we show that cell-free manipulations may underestimate allelopathy at low cell densities if effects are transmitted during cell-cell interactions. However, we suggest that the evolution of allelopathy under field conditions may be unlikely even if based on cell-cell interactions. The spatial dispersion of cells in turbulent flow will make it difficult for an allelopathic cell to receive an exclusive benefit, and a dispersion model shows that dividing cells are rapidly separated constraining clone selection. Instead, we propose that reported allelopathic effects may be nonadaptive side effects of predator-prey or casual parasitic cell-cell interactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

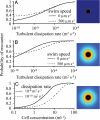
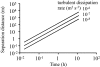
References
-
- Hallegraeff GM. A review of harmful algal blooms and their apparent global increase. Phycologia. 1993;32:79–99.
-
- Turner JT, Tester PA, Hansen PJ. In: Physiological Ecology of Harmful Algal Blooms. Anderson DM, Cembella AD, Hallegraeff GM, editors. Berlin: Springer; 1998.
-
- Bertin C, Yang X, Weston LA. The role of root exudates and allelochemicals in the rhizosphere. Plant Soil. 2003;256:67–83.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

