Therapeutics by cytotoxic metabolite accumulation: pemetrexed causes ZMP accumulation, AMPK activation, and mammalian target of rapamycin inhibition
- PMID: 19549896
- PMCID: PMC2706929
- DOI: 10.1158/0008-5472.CAN-08-4979
Therapeutics by cytotoxic metabolite accumulation: pemetrexed causes ZMP accumulation, AMPK activation, and mammalian target of rapamycin inhibition
Abstract
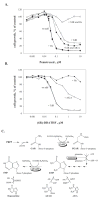
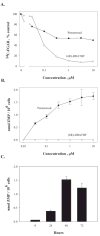
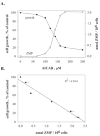
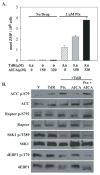
Pemetrexed represents the first antifolate cancer drug to be approved by the Food and Drug Administration in 20 years; it is currently in widespread use for first line therapy of mesothelioma and non-small cell lung cancer. Pemetrexed has more than one site of action; the primary site is thymidylate synthase. We now report that the secondary target is the downstream folate-dependent enzyme in de novo purine synthesis, aminoimidazolecarboxamide ribonucleotide formyltransferase (AICART). The substrate of the AICART reaction, ZMP, accumulated in intact pemetrexed-inhibited tumor cells, identifying AICART as the step in purine synthesis that becomes rate-limiting after drug treatment. The accumulating ZMP causes an activation of AMP-activated protein kinase with subsequent inhibition of the mammalian target of rapamycin (mTOR) and hypophosphorylation of the downstream targets of mTOR that control initiation of protein synthesis and cell growth. We suggest that the activity of pemetrexed against human cancers is a reflection of its direct inhibition of folate-dependent target proteins combined with prolonged inhibition of the mTOR pathway secondary to accumulation of ZMP.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Habeck LL, Mendelsohn LG, Shih C, et al. Substrate specificity of mammalian folylpolyglutamate synthetase for 5,10-dideazatetrahydrofolate analogs. Mol Pharmacol. 1995;48:326–33. - PubMed
-
- Chattopadhyay S, Moran RG, Goldman ID. Pemetrexed: biochemical and cellular pharmacology, mechanisms, and clinical applications. Mol Cancer Ther. 2007;6:404–17. - PubMed
-
- Taylor EC, Hamby JM, Shih C, et al. Synthesis and antitumor activity of 5-deaza-5,6,7,8-tetrahydrofolic acid and its N10-substituted analogues. J Med Chem. 1989;32:1517–22. - PubMed
-
- Shih C, Chen VJ, Gossett LS, et al. LY231514, a pyrrolo[2,3-d]pyrimidine-based antifolate that inhibits multiple folate-requiring enzymes. Cancer Res. 1997;57:1116–23. - PubMed
-
- Chattopadhyay S, Zhao R, Krupenko SA, Krupenko N, Goldman ID. The inverse relationship between reduced folate carrier function and pemetrexed activity in a human colon cancer cell line. Mol Cancer Ther. 2006;5:438–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

