Stimulating the GPR30 estrogen receptor with a novel tamoxifen analogue activates SF-1 and promotes endometrial cell proliferation
- PMID: 19549922
- PMCID: PMC2756996
- DOI: 10.1158/0008-5472.CAN-08-1622
Stimulating the GPR30 estrogen receptor with a novel tamoxifen analogue activates SF-1 and promotes endometrial cell proliferation
Abstract
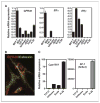
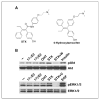
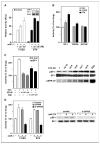
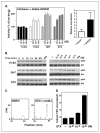
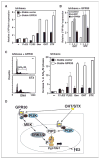
Estrogens and selective estrogen receptor (ER) modulators such as tamoxifen are known to increase uterine cell proliferation. Mounting evidence suggests that estrogen signaling is mediated not only by ERalpha and ERbeta nuclear receptors, but also by GPR30 (GPER), a seven transmembrane (7TM) receptor. Here, we report that primary human endometriotic H-38 cells express high levels of GPR30 with no detectable ERalpha or ERbeta. Using a novel tamoxifen analogue, STX, which activates GPR30 but not ERs, significant stimulation of the phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) pathways was observed in H-38 cells and in Ishikawa endometrial cancer cells expressing GPR30; a similar effect was observed in JEG3 choriocarcinoma cells. STX treatment also increased cellular pools of phosphatidylinositol (3,4,5) triphosphate, a proposed ligand for the nuclear hormone receptor SF-1 (NR5A1). Consistent with these findings, STX, tamoxifen, and the phytoestrogen genistein were able to increase SF-1 transcription, promote Ishikawa cell proliferation, and induce the SF-1 target gene aromatase in a GPR30-dependent manner. Our findings suggest a novel signaling paradigm that is initiated by estrogen activation of the 7TM receptor GPR30, with signal transduction cascades (PI3K and MAPK) converging on nuclear hormone receptors (SF-1/LRH-1) to modulate their transcriptional output. We propose that this novel GPR30/SF-1 pathway increases local concentrations of estrogen, and together with classic ER signaling, mediate the proliferative effects of synthetic estrogens such as tamoxifen, in promoting endometriosis and endometrial cancers.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures

Similar articles
-
Estradiol and tamoxifen induce cell migration through GPR30 and activation of focal adhesion kinase (FAK) in endometrial cancers with low or without nuclear estrogen receptor α (ERα).PLoS One. 2013 Sep 9;8(9):e72999. doi: 10.1371/journal.pone.0072999. eCollection 2013. PLoS One. 2013. PMID: 24039841 Free PMC article.
-
The G protein-coupled receptor GPR30 mediates the nontranscriptional effect of estrogen on the activation of PI3K/Akt pathway in endometrial cancer cells.Int J Gynecol Cancer. 2013 Jan;23(1):52-9. doi: 10.1097/IGC.0b013e31827912b8. Int J Gynecol Cancer. 2013. PMID: 23235274
-
Estrogenic G protein-coupled receptor 30 signaling is involved in regulation of endometrial carcinoma by promoting proliferation, invasion potential, and interleukin-6 secretion via the MEK/ERK mitogen-activated protein kinase pathway.Cancer Sci. 2009 Jun;100(6):1051-61. doi: 10.1111/j.1349-7006.2009.01148.x. Epub 2009 Mar 9. Cancer Sci. 2009. PMID: 19432902 Free PMC article.
-
Estrogen receptors as potential therapeutic target in endometrial cancer.J Recept Signal Transduct Res. 2023 Feb;43(1):19-26. doi: 10.1080/10799893.2023.2187643. Epub 2023 Mar 8. J Recept Signal Transduct Res. 2023. PMID: 36883690 Review.
-
G protein-coupled receptor 30 in tumor development.Endocrine. 2010 Aug;38(1):29-37. doi: 10.1007/s12020-010-9363-z. Epub 2010 Jul 8. Endocrine. 2010. PMID: 20960099 Review.
Cited by
-
Stimulation of GPR30 increases release of EMMPRIN-containing microvesicles in human uterine epithelial cells.J Clin Endocrinol Metab. 2012 Dec;97(12):4613-22. doi: 10.1210/jc.2012-2098. Epub 2012 Sep 25. J Clin Endocrinol Metab. 2012. PMID: 23012390 Free PMC article.
-
Role of oestrogen receptors in bladder cancer development.Nat Rev Urol. 2013 Jun;10(6):317-26. doi: 10.1038/nrurol.2013.53. Epub 2013 Apr 16. Nat Rev Urol. 2013. PMID: 23588401 Review.
-
Activation of GPR30 inhibits cardiac fibroblast proliferation.Mol Cell Biochem. 2015 Jul;405(1-2):135-48. doi: 10.1007/s11010-015-2405-3. Epub 2015 Apr 17. Mol Cell Biochem. 2015. PMID: 25893735 Free PMC article.
-
Endometriosis and nuclear receptors.Hum Reprod Update. 2019 Jul 1;25(4):473-485. doi: 10.1093/humupd/dmz005. Hum Reprod Update. 2019. PMID: 30809650 Free PMC article. Review.
-
Differential expression of upstream stimulatory factor (USF) 2 variants in eutopic endometria from women with endometriosis: estradiol regulation.Biol Res. 2015 Oct 9;48:56. doi: 10.1186/s40659-015-0047-2. Biol Res. 2015. PMID: 26453052 Free PMC article.
References
-
- Levin ER, Pietras RJ. Estrogen receptors outside the nucleus in breast cancer. Breast Cancer Res Treat. 2008;108:351–61. - PubMed
-
- Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Ann Rev Physiol. 2008;70:165–90. - PubMed
-
- Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006;20:1996–2009. - PubMed
-
- Harrington WR, Kim SH, Funk CC, et al. Estrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of estrogen action. Mol Endocrinol. 2006;20:491–502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

