Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility
- PMID: 19551144
- PMCID: PMC2696084
- DOI: 10.1371/journal.pone.0006026
Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility
Abstract
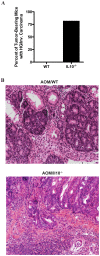
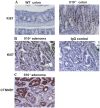
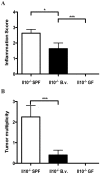
It is well established that the intestinal microbiota plays a key role in the pathogenesis of Crohn's disease (CD) and ulcerative colitis (UC) collectively referred to as inflammatory bowel disease (IBD). Epidemiological studies have provided strong evidence that IBD patients bear increased risk for the development of colorectal cancer (CRC). However, the impact of the microbiota on the development of colitis-associated cancer (CAC) remains largely unknown. In this study, we established a new model of CAC using azoxymethane (AOM)-exposed, conventionalized-Il10(-/-) mice and have explored the contribution of the host intestinal microbiota and MyD88 signaling to the development of CAC. We show that 8/13 (62%) of AOM-Il10(-/-) mice developed colon tumors compared to only 3/15 (20%) of AOM- wild-type (WT) mice. Conventionalized AOM-Il10(-/-) mice developed spontaneous colitis and colorectal carcinomas while AOM-WT mice were colitis-free and developed only rare adenomas. Importantly, tumor multiplicity directly correlated with the presence of colitis. Il10(-/-) mice mono-associated with the mildly colitogenic bacterium Bacteroides vulgatus displayed significantly reduced colitis and colorectal tumor multiplicity compared to Il10(-/-) mice. Germ-free AOM-treated Il10(-/-) mice showed normal colon histology and were devoid of tumors. Il10(-/-); Myd88(-/-) mice treated with AOM displayed reduced expression of Il12p40 and Tnfalpha mRNA and showed no signs of tumor development. We present the first direct demonstration that manipulation of the intestinal microbiota alters the development of CAC. The TLR/MyD88 pathway is essential for microbiota-induced development of CAC. Unlike findings obtained using the AOM/DSS model, we demonstrate that the severity of chronic colitis directly correlates to colorectal tumor development and that bacterial-induced inflammation drives progression from adenoma to invasive carcinoma.
Conflict of interest statement
Figures


References
-
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. - PubMed
-
- Mantovani A. Cancer: inflammation by remote control. Nature. 2005;435:752–753. - PubMed
-
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. - PubMed
-
- Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

