Raf Kinase Inhibitory Protein protects cells against locostatin-mediated inhibition of migration
- PMID: 19551145
- PMCID: PMC2696091
- DOI: 10.1371/journal.pone.0006028
Raf Kinase Inhibitory Protein protects cells against locostatin-mediated inhibition of migration
Abstract
Background: Raf Kinase Inhibitory Protein (RKIP, also PEBP1), a member of the Phosphatidylethanolamine Binding Protein family, negatively regulates growth factor signaling by the Raf/MAP kinase pathway. Since an organic compound, locostatin, was reported to bind RKIP and inhibit cell migration by a Raf-dependent mechanism, we addressed the role of RKIP in locostatin function.
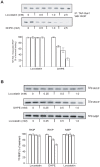
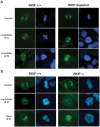
Methods/findings: We analyzed locostatin interaction with RKIP and examined the biological consequences of locostatin binding on RKIP function. NMR studies show that a locostatin precursor binds to the conserved phosphatidylethanolamine binding pocket of RKIP. However, drug binding to the pocket does not prevent RKIP association with its inhibitory target, Raf-1, nor affect RKIP phosphorylation by Protein Kinase C at a regulatory site. Similarly, exposure of wild type, RKIP-depleted HeLa cells or RKIP-deficient (RKIP(-/-)) mouse embryonic fibroblasts (MEFs) to locostatin has no effect on MAP kinase activation. Locostatin treatment of wild type MEFs causes inhibition of cell migration following wounding. RKIP deficiency impairs migration further, indicating that RKIP protects cells against locostatin-mediated inhibition of migration. Locostatin treatment of depleted or RKIP(-/-) MEFs reveals cytoskeletal disruption and microtubule abnormalities in the spindle.
Conclusions/significance: These results suggest that locostatin's effects on cytoskeletal structure and migration are caused through mechanisms independent of its binding to RKIP and Raf/MAP kinase signaling. The protective effect of RKIP against drug inhibition of migration suggests a new role for RKIP in potentially sequestering toxic compounds that may have deleterious effects on cells.
Conflict of interest statement
Figures





References
-
- Zeng L, Imamoto A, Rosner MR. Raf kinase inhibitory protein (RKIP): a physiological regulator and future therapeutic target. Expert Opin Ther Targets. 2008;12:1275–1287. - PubMed
-
- Granovsky AE, Rosner MR. Raf kinase inhibitory protein: a signal transduction modulator and metastasis suppressor. Cell Res. 2008;18:452–457. - PubMed
-
- Yeung K, Seitz T, Li S, Janosch P, McFerran B, et al. Suppression of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature. 1999;401:173–177. - PubMed
-
- Corbit KC, Trakul N, Eves EM, Diaz B, Marshall M, et al. Activation of Raf-1 Signaling by Protein Kinase C through a Mechanism Involving Raf Kinase Inhibitory Protein. J Biol Chem. 2003;278:13061–13068. - PubMed
-
- Trakul N, Menard RE, Schade GR, Qian Z, Rosner MR. Raf kinase inhibitory protein regulates Raf-1 but not B-Raf kinase activation. J Biol Chem. 2005;280:24931–24940. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

