Multiple isotopic labels for quantitative mass spectrometry
- PMID: 19551992
- PMCID: PMC2771887
- DOI: 10.1021/ac801654h
Multiple isotopic labels for quantitative mass spectrometry
Abstract
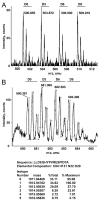
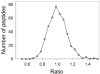
Quantitative mass spectrometry is often performed using isotopically labeled samples. Although the 4-trimethylammoniumbutyryl (TMAB) labels have many advantages over other isotopic tags, only two forms have previously been synthesized (i.e., a heavy form containing nine deuteriums and a light form without deuterium). In the present report, two additional forms containing three and six deuteriums have been synthesized and tested. These additional isotopic tags perform identically to the previously reported tags; peptides labeled with the new TMAB reagents coelute from reversed-phase HPLC columns with peptides labeled with the lighter and heavier TMAB reagents. Altogether, these four tags allow for multivariate analysis in a single liquid chromatography/mass spectrometry analysis, with each isotopically tagged peptide differing in mass by 3 Da per tag incorporated. The synthetic scheme is described in simple terms so that a biochemist without specific training in organic chemistry can perform the synthesis. The interpretation of tandem mass spectrometry data for the TMAB-labeled peptides is also described in more detail. The additional TMAB isotopic reagents described here, together with the additional description of the synthesis and analysis, should allow these labels to be more widely used for proteomics and peptidomics analyses.
Figures




References
-
- Strand FL. Prog Drug Res. 2003;61:1–37. - PubMed
-
- Hokfelt T, Broberger C, Xu ZQ, Sergeyev V, Ubink R, Diez M. Neuropharm. 2000;39:1337–56. - PubMed
-
- Hummon AB, Amare A, Sweedler JV. Mass Spectrom Rev. 2006;25:77–98. - PubMed
-
- Hummon AB, Richmond TA, Verleyen P, Baggerman G, Huybrechts J, Ewing MA, Vierstraete E, Rodriguez-Zas SL, Schoofs L, Robinson GE, Sweedler JV. Science. 2006;314:647–49. - PubMed
-
- Yuan X, Desiderio DM. J Mass Spectrom. 2005;40:176–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

