Targeting and insertion of the cholesterol-binding translocator protein into the outer mitochondrial membrane
- PMID: 19552401
- PMCID: PMC2748833
- DOI: 10.1021/bi900854z
Targeting and insertion of the cholesterol-binding translocator protein into the outer mitochondrial membrane
Abstract
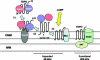
Translocator protein (18 kDa, TSPO), previously known as the peripheral-type benzodiazepine receptor, is an outer mitochondrial membrane (OMM) protein necessary for cholesterol import and steroid production. We reconstituted the mitochondrial targeting and insertion of TSPO into the OMM to analyze the signals and mechanisms required for this process. Initial studies indicated the formation of a mitochondrial 66 kDa complex through Blue Native-PAGE analysis. The formation of this complex was found to be dependent on the presence of ATP and the cytosolic chaperone Hsp90. Through mutational analysis we identified two areas necessary for TSPO targeting, import, and function: amino acids 103-108 (Schellman motif), which provide the necessary structural orientation for import, and the cholesterol-binding C-terminus required for insertion. Although the translocase of the outer mitochondrial membrane (TOM) complex proteins Tom22 and Tom40 were present in the OMM, the TOM complex did not interact with TSPO. In search of proteins involved in TSPO import, we analyzed complexes known to interact with TSPO by mass spectrometry. Formation of the 66 kDa complex was found to be dependent on an identified protein, Metaxin 1, for formation and TSPO import. The level of import of TSPO into steroidogenic cell mitochondria was increased following treatment of the cells with cAMP. These findings suggest that the initial targeting of TSPO to mitochondria is dependent upon the presence of cytosolic chaperones interacting with the import receptor Tom70. The C-terminus plays an important role in targeting TSPO to mitochondria, whereas its import into the OMM is dependent upon the presence of the Schellman motif. Final integration of TSPO into the OMM occurs via its interaction with Metaxin 1. Import of TSPO into steroidogenic cell mitochondria is regulated by cAMP.
Figures






References
-
- Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapere JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006;27:402–409. - PubMed
-
- Lacapere JJ, Papadopoulos V. Peripheral-type benzodiazepine receptor: structure and function of a cholesterol-binding protein in steroid and bile acid biosynthesis. Steroids. 2003;68:569–585. - PubMed
-
- Murail S, Robert JC, Coic YM, Neumann JM, Ostuni MA, Yao ZX, Papadopoulos V, Jamin N, Lacapere JJ. Secondary and tertiary structures of the transmembrane domains of the translocator protein TSPO determined by NMR. Stabilization of the TSPO tertiary fold upon ligand binding. Biochim. Biophys. Acta. 2008;1778:1375–1381. - PubMed
-
- Garnier M, Dimchev AB, Boujrad N, Price JM, Musto NA, Papadopoulos V. In vitro reconstitution of a functional peripheral-type benzodiazepine receptor from mouse Leydig tumor cells. Mol. Pharmacol. 1994;45:201–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

