Palladium- and copper-catalyzed arylation of carbon-hydrogen bonds
- PMID: 19552413
- PMCID: PMC2846291
- DOI: 10.1021/ar9000058
Palladium- and copper-catalyzed arylation of carbon-hydrogen bonds
Abstract
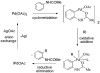
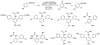
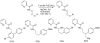
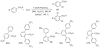
The transition-metal-catalyzed functionalization of C-H bonds is a powerful method for generating carbon-carbon bonds. Although significant advances to this field have been reported during the past decade, many challenges remain. First, most of the methods are substrate-specific and thus cannot be generalized. Second, conversions of unactivated (i.e., not benzylic or alpha to heteroatom) sp(3) C-H bonds to C-C bonds are rare, with most examples limited to t-butyl groups, a conversion that is inherently simple because there are no beta-hydrogens that can be eliminated. Finally, the palladium, rhodium, and ruthenium catalysts routinely used for the conversion of C-H bonds to C-C bonds are expensive. Catalytically active metals that are cheaper and less exotic (e.g., copper, iron, and manganese) are rarely used. This Account describes our attempts to provide solutions to these three problems. We have developed a general method for directing-group-containing arene arylation by aryl iodides. Using palladium acetate as the catalyst, we arylated anilides, benzamides, benzoic acids, benzylamines, and 2-substituted pyridine derivatives under nearly identical conditions. We have also developed a method for the palladium-catalyzed auxiliary-assisted arylation of unactivated sp(3) C-H bonds. This procedure allows for the beta-arylation of carboxylic acid derivatives and the gamma-arylation of amine derivatives. Furthermore, copper catalysis can be used to mediate the arylation of acidic arene C-H bonds (i.e., those with pK(a) values <35 in DMSO). Using a copper iodide catalyst in combination with a base and a phenanthroline ligand, we successfully arylated electron-rich and electron-deficient heterocycles and electron-poor arenes possessing at least two electron-withdrawing groups. The reaction exhibits unusual regioselectivity: arylation occurs at the most hindered position. This copper-catalyzed method supplements the well-known C-H activation/borylation methodology, in which functionalization usually occurs at the least hindered position. We also describe preliminary investigations to determine the mechanisms of these transformations. We anticipate that other transition metals, including iron, nickel, cobalt, and silver, will also be able to facilitate deprotonation/arylation reaction sequences.
Figures











References
-
-
Recent reviews: Seregin IV, Gevorgyan V. Direct Transition Metal-Catalyzed Functionalization of Heteroaromatic Compounds. Chem. Soc. Rev. 2007;36:1173–1193. Alberico D, Scott ME, Lautens M. Aryl-Aryl Bond Formation by Transition-Metal-Catalyzed Direct Arylation. Chem. Rev. 2007;107:174–238. Ackermann L. Catalytic Arylations with Challenging Substrates: from Air-Stable HASPO Preligands to Indole Syntheses and C-H Bond Functionalizations. Synlett. 2007:507–526. Campeau L-C, Fagnou K. Applications of and Alternatives to π-Electron-Deficient Azine Organometallics in Metal Catalyzed Cross-Coupling Reactions. Chem. Soc. Rev. 2007;36:1058–1068. Lewis JC, Bergman RG, Ellman JA. Direct Functionalization of Nitrogen Heterocycles via Rh-Catalyzed C-H Bond Activation. Acc. Chem. Res. 2008;41:1013–1025.
-
-
- Kakiuchi F, Kan S, Igi K, Chatani N, Murai S. A Ruthenium-Catalyzed Reaction of Aromatic Ketones with Arylboronates: A New Method for the Arylation of Aromatic Compounds via C-H Bond Cleavage. J. Am. Chem. Soc. 2003;125:1698–1699. - PubMed
- Oi S, Fukita S, Inoue Y. Rhodium-Catalyzed Direct ortho-Arylation of 2-Arylpyridines with Arylstannanes via C-H Activation. Chem. Commun. 1998:2439–2440.
- Bedford RB, Coles SJ, Hursthouse MB, Limmert ME. The Catalytic Intermolecular ortho-Arylation of Phenols. Angew. Chem., Int. Ed. 2003;42:112–114. - PubMed
- Caron L, Campeau L-C, Fagnou K. Palladium-Catalyzed Direct Arylation of Nitro-Substituted Aromatics with Aryl Halides. Org. Lett. 2008;10:4533–4536. - PubMed
- Cho SH, Hwang SJ, Chang S. Palladium-Catalyzed C-H Functionalization of Pyridine N-Oxides: Highly Selective Alkenylation and Direct Arylation with Unactivated Arenes. J. Am. Chem. Soc. 2008;130:9254–9256. - PubMed
- Chen X, Li J-J, Hao X-S, Goodhue CE, Yu J-Q. Palladium-Catalyzed Alkylation of Aryl C-H Bonds with sp3 Organotin Reagents Using Benzoquinone as a Crucial Promoter. J. Am. Chem. Soc. 2006;128:78–79. - PubMed
-
- Pivsa-Art S, Satoh T, Kawamura Y, Miura M, Nomura M. Palladium-Catalyzed Arylation of Azole Compounds with Aryl Halides in the Presence of Alkali Metal Carbonates and the Use of Copper Iodide in the Reaction. Bull. Chem. Soc. Jpn. 1998;71:467–473.
- Park C-H, Ryabova V, Seregin IV, Sromek AW, Gevorgyan V. Palladium-Catalyzed Arylation and Heteroarylation of Indolizines. Org. Lett. 2004;6:1159–1162. - PMC - PubMed
- Bellina F, Cauteruccio S, Mannina L, Rossi R, Viel S. Regioselective Synthesis of 1,5-Diaryl-1H-Imidazoles by Palladium-Catalyzed Direct Arylation of 1-Aryl-1H-Imidazoles. J. Org. Chem. 2005;70:3997–4005. - PubMed
- Flegeau EF, Popkin ME, Greaney MF. Direct Arylation of Oxazoles at C2. A Concise Approach to Consecutively Linked Oxazoles. Org. Lett. 2008;10:2717–2720. - PubMed
- Iwasaki M, Yorimitsu H, Oshima K. Microwave-Assisted Palladium-Catalyzed Direct Arylation of 1,4-Disubstituted 1,2,3-Triazoles with Aryl Chlorides. Chem. -Asian J. 2007;2:1430–1435. - PubMed
-
- Chaumontet M, Piccardi R, Baudoin O. Synthesis of 3,4-Dihydroisoquinolines by a C(sp3)-H Activation/Electrocyclization Strategy: Total Synthesis of Coralydine. Angew. Chem. Int. Ed. 2009;48:179–182. - PubMed
- Lafrance M, Gorelsky SI, Fagnou K. High-Yielding Palladium-Catalyzed Intramolecular Alkane Arylation: Reaction Development and Mechanistic Studies. J. Am. Chem. Soc. 2007;129:14570–14571. - PubMed
- Barder TE, Walker SD, Martinelli JR, Buchwald SL. Catalysts for Suzuki-Miyaura Coupling Processes: Scope and Studies of the Effect of Ligand Structure. J. Am. Chem. Soc. 2005;127:4685–4696. - PubMed
- Dyker G. Palladium-Catalyzed C-H Activation of tert-Butyl Groups: A Simple Synthesis of 1,2-Dihydrocyclobutabenzene Derivatives. Angew. Chem. Int. Ed. 1994;33:103–105.
- Giri R, Maugel N, Li J-J, Wang D-H, Breazzano SP, Saunders LB, Yu J-Q. Palladium-Catalyzed Methylation and Arylation of sp2 and sp3 C-H Bonds in Simple Carboxylic Acids. J. Am. Chem. Soc. 2007;129:3510–3511. - PubMed
- Wang D-H, Wasa M, Giri R, Yu J-Q. Pd(II)-Catalyzed Cross-Coupling of sp3 C-H Bonds with sp2 and sp3 Boronic Acids Using Air as the Oxidant. J. Am. Chem. Soc. 2008;130:7190–7191. - PubMed
-
- Brasche G, Buchwald SL. C-H Functionalization/C-N Bond Formation: Copper-Catalyzed Synthesis of Benzimidazoles from Amidines. Angew. Chem., Int. Ed. 2008;47:1932–1934. - PubMed
- Norinder J, Matsumoto A, Yoshikai N, Nakamura E. Iron-Catalyzed Direct Arylation through Directed C-H Bond Activation. J. Am. Chem. Soc. 2008;130:5858–5859. - PubMed
- Chen X, Hao X-S, Goodhue CE, Yu J-Q. Cu(II)-Catalyzed Functionalizations of Aryl C-H Bonds Using O2 as an Oxidant. J. Am. Chem. Soc. 2006;128:6790–6791. - PubMed
- Uemura T, Imoto S, Chatani N. Amination of the ortho C-H Bonds by the Cu(OAc)2-Mediated Reaction of 2-Phenylpyridines with Anilines. Chem. Lett. 2006;35:842–843.
- Phipps RJ, Grimster NP, Gaunt MJ. Cu(Ii)-Catalyzed Direct And Site-Selective Arylation of Indoles Under Mild Conditions. J. Am. Chem. Soc. 2008;130:8172–8174. - PubMed
- Wen J, Zhang J, Chen S-Y, Li J, Yu X-Q. Iron-Mediated Direct Arylation of Unactivated Arenes. Angew. Chem., Int. Ed. 2008;47:8897–8900. - PubMed
- Ueda S, Nagasawa H. Synthesis of 2-Arylbenzoxazoles by Copper-Catalyzed Intramolecular Oxidative C-O Coupling of Benzanilides. Angew. Chem., Int. Ed. 2008;47:6411–6413. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

