Lipid-shelled vehicles: engineering for ultrasound molecular imaging and drug delivery
- PMID: 19552457
- PMCID: PMC2727628
- DOI: 10.1021/ar8002442
Lipid-shelled vehicles: engineering for ultrasound molecular imaging and drug delivery
Abstract
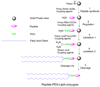
Ultrasound pressure waves can map the location of lipid-stabilized gas micro-bubbles after their intravenous administration in the body, facilitating an estimate of vascular density and microvascular flow rate. Microbubbles are currently approved by the Food and Drug Administration as ultrasound contrast agents for visualizing opacification of the left ventricle in echocardiography. However, the interaction of ultrasound waves with intravenously-injected lipid-shelled particles, including both liposomes and microbubbles, is a far richer field. Particles can be designed for molecular imaging and loaded with drugs or genes; the mechanical and thermal properties of ultrasound can then effect localized drug release. In this Account, we provide an overview of the engineering of lipid-shelled microbubbles (typical diameter 1000-10 000 nm) and liposomes (typical diameter 65-120 nm) for ultrasound-based applications in molecular imaging and drug delivery. The chemistries of the shell and core can be optimized to enhance stability, circulation persistence, drug loading and release, targeting to and fusion with the cell membrane, and therapeutic biological effects. To assess the biodistribution and pharmacokinetics of these particles, we incorporated positron emission tomography (PET) radioisotopes on the shell. The radionuclide (18)F (half-life approximately 2 h) was covalently coupled to a dipalmitoyl lipid, followed by integration of the labeled lipid into the shell, facilitating short-term analysis of particle pharmacokinetics and metabolism of the lipid molecule. Alternately, labeling a formed particle with (64)Cu (half-life 12.7 h), after prior covalent incorporation of a copper-chelating moiety onto the lipid shell, permits pharmacokinetic study of particles over several days. Stability and persistence in circulation of both liposomes and microbubbles are enhanced by long acyl chains and a poly(ethylene glycol) coating. Vascular targeting has been demonstrated with both nano- and microdiameter particles. Targeting affinity of the microbubble can be modulated by burying the ligand within a polymer brush layer; the application of ultrasound then reveals the ligand, enabling specific targeting of only the insonified region. Microbubbles and liposomes require different strategies for both drug loading and release. Microbubble loading is inhibited by the gas core and enhanced by layer-by-layer construction or conjugation of drug-entrapped particles to the surface. Liposome loading is typically internal and is enhanced by drug-specific loading techniques. Drug release from a microbubble results from the oscillation of the gas core diameter produced by the sound wave, whereas that from a liposome is enhanced by heat produced from the local absorption of acoustic energy within the tissue microenvironment. Biological effects induced by ultrasound, such as changes in cell membrane and vascular permeability, can enhance drug delivery. In particular, as microbubbles oscillate near a vessel wall, shock waves or liquid jets enhance drug transport. Mild heating induced by ultrasound, either before or after injection of the drug, facilitates the transport of liposomes from blood vessels to the tissue interstitium, thus increasing drug accumulation in the target region. Lipid-shelled vehicles offer many opportunities for chemists and engineers; ultrasound-based applications beyond the few currently in common use will undoubtedly soon multiply as molecular construction techniques are further refined.
Figures





Similar articles
-
A novel nested liposome drug delivery vehicle capable of ultrasound triggered release of its payload.J Control Release. 2011 Nov 7;155(3):358-66. doi: 10.1016/j.jconrel.2011.06.032. Epub 2011 Jul 2. J Control Release. 2011. PMID: 21745505 Free PMC article.
-
Italian Society of Cardiovascular Echography (SIEC) Consensus Conference on the state of the art of contrast echocardiography.Ital Heart J. 2004 Apr;5(4):309-34. Ital Heart J. 2004. PMID: 15185894 Review.
-
Dynamic microPET imaging of ultrasound contrast agents and lipid delivery.J Control Release. 2008 Nov 12;131(3):160-6. doi: 10.1016/j.jconrel.2008.07.030. Epub 2008 Jul 29. J Control Release. 2008. PMID: 18718854 Free PMC article.
-
QCM-D Investigations on Cholesterol-DNA Tethering of Liposomes to Microbubbles for Therapy.J Phys Chem B. 2023 Mar 23;127(11):2466-2474. doi: 10.1021/acs.jpcb.2c07256. Epub 2023 Mar 14. J Phys Chem B. 2023. PMID: 36917458 Free PMC article.
-
Ultrasound contrast microbubbles in imaging and therapy: physical principles and engineering.Phys Med Biol. 2009 Mar 21;54(6):R27-57. doi: 10.1088/0031-9155/54/6/R01. Epub 2009 Feb 19. Phys Med Biol. 2009. PMID: 19229096 Free PMC article. Review.
Cited by
-
Synthesis and physicochemical evaluation of fluorinated lipopeptide precursors of ligands for microbubble targeting.Beilstein J Org Chem. 2021 Feb 19;17:511-518. doi: 10.3762/bjoc.17.45. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 33727974 Free PMC article.
-
Cyanine 5.5 conjugated nanobubbles as a tumor selective contrast agent for dual ultrasound-fluorescence imaging in a mouse model.PLoS One. 2013 Apr 18;8(4):e61224. doi: 10.1371/journal.pone.0061224. Print 2013. PLoS One. 2013. PMID: 23637799 Free PMC article.
-
Phase separation behavior of mixed lipid systems at neutral and low pH: coarse-grained simulations with DMD/LIME.Langmuir. 2015 Jan 27;31(3):1086-94. doi: 10.1021/la504082x. Epub 2015 Jan 15. Langmuir. 2015. PMID: 25549801 Free PMC article.
-
Spontaneous shape reconfigurations in multicompartmental microcylinders.Proc Natl Acad Sci U S A. 2012 Oct 2;109(40):16057-62. doi: 10.1073/pnas.1213669109. Epub 2012 Sep 19. Proc Natl Acad Sci U S A. 2012. PMID: 22992652 Free PMC article.
-
Micro/nano-bubble-assisted ultrasound to enhance the EPR effect and potential theranostic applications.Theranostics. 2020 Jan 1;10(2):462-483. doi: 10.7150/thno.37593. eCollection 2020. Theranostics. 2020. PMID: 31903132 Free PMC article. Review.
References
-
- Serfis AB, Katzenberger R, Williams K, Tran N. Association of blood clotting factors I and VII with phospholipid monolayers at the air-water interface. J. Colloid Interface Sci. 1999;215:356–363. - PubMed
-
- Morgan KE, Allen JS, Dayton PA, Chomas JE, Klibanov AL, Ferrara KW. Experimental and theoretical evaluation of microbubble behavior: Effect of transmitted phase and bubble size. Ieee Transactions on Ultrasonics Ferroelectrics and Frequency Control. 2000;47:1494–1509. - PubMed
-
- Kaul S. Myocardial Contrast Echocardiography: A 25-Year Retrospective. Circulation. 2008;118:291–308. - PubMed
-
- Yatvin M, Weinstein JN, Dennis WH, Blumenthal R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science. 1978;202:1290–1293. - PubMed
-
- Drummond D, Noble CO, Hayes ME, Park JW, Kirpotin DB. Pharmacokinetics and in vivo drug release rates in liposomal nanocarrier development. Journal of Pharmaceutical Sciences. 2008;97:4696–4740. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

