A low dose of Mycoplasma pneumoniae infection enhances an established allergic inflammation in mice: the role of the prostaglandin E2 pathway
- PMID: 19552640
- PMCID: PMC2784117
- DOI: 10.1111/j.1365-2222.2009.03309.x
A low dose of Mycoplasma pneumoniae infection enhances an established allergic inflammation in mice: the role of the prostaglandin E2 pathway
Abstract
Background: Over 40% of chronic stable asthma patients have evidence of respiratory Mycoplasma pneumoniae (Mp) infection as detected by PCR, but not by serology and culture, suggesting that a low-level Mp is involved in chronic asthma. However, the role of such a low-level Mp infection in the regulation of allergic inflammation remains unknown.
Objective: To determine the impact of a low-level Mp infection in mice with established airway allergic inflammation on allergic responses such as eosinophilia and chemokine eotaxin-2, and the underlying mechanisms [i.e. the prostaglandin E(2) (PGE(2)) pathway] since PGE(2) inhalation before an allergen challenge suppressed the eosinophil infiltration in human airways.
Methods: BALB/c mouse models of ovalbumin (OVA)-induced allergic asthma with an ensuing low- or high-dose Mp were used to assess IL-4 expression, bronchoalveolar lavage (BAL) eosinophil, eotaxin-2 and PGE(2) levels, and lung mRNA levels of microsomal prostaglandin E synthase-1 (mPGES-1). Primary alveolar macrophages (pAMs) from naïve BALB/c mice were cultured to determine whether Mp-induced PGE(2) or exogenous PGE(2) down-regulates IL-4/IL-13-induced eotaxin-2.
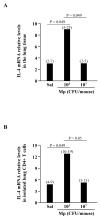
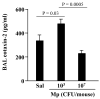
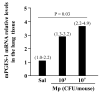
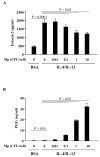
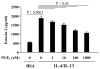
Results: Low-dose Mp in allergic mice significantly enhanced IL-4 and eotaxin-2, and moderately promoted lung eosinophilia, whereas high-dose Mp significantly reduced lung eosinophilia and tended to decrease IL-4 and eotaxin-2. Moreover, in both OVA-naïve and allergic mice, lung mPGES-1 mRNA and BAL PGE(2) levels were elevated in mice infected with high-dose, but not low-dose Mp. In pAMs, IL-4/IL-13 significantly increased eotaxin-2, which was reduced by Mp infection accompanied by dose-dependent PGE(2) induction. Exogenous PGE(2) inhibited IL-4/IL-13-induced eotaxin-2 in a dose-dependent manner.
Conclusions: This study highlights a novel concept on how different bacterial loads in the lung modify the established allergic airway inflammation and thus interact with an allergen to further induce Th2 responses. That is, unlike high-level Mp, low-level Mp fails to effectively induce PGE(2) to down-regulate allergic responses (e.g. eotaxin-2), thus maintaining or even worsening allergic inflammation in asthmatic airways.
Figures
References
-
- Martin RJ, Kraft M, Chu HW, Berns EA, Cassell GH. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001;107:595–601. - PubMed
-
- Kraft M, Cassell GH, Pak J, Martin RJ. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest. 2002;121:1782–8. - PubMed
-
- Gil JC, Cedillo RL, Mayagoitia BG, Paz MD. Isolation of Mycoplasma pneumoniae from asthmatic patients. Ann Allergy. 1993;70:23–5. - PubMed
-
- Chang YT, Yang YH, Chiang BL. The significance of a rapid cold hemagglutination test for detecting mycoplasma infections in children with asthma exacerbation. J Microbiol Immunol Infect. 2006;39:28–32. - PubMed
-
- Black PN. Antibiotics for the treatment of asthma. Curr Opin Pharmacol. 2007;7:266–71. - PubMed

