Messenger RNA export from the nucleus: a series of molecular wardrobe changes
- PMID: 19552647
- PMCID: PMC3702165
- DOI: 10.1111/j.1600-0854.2009.00944.x
Messenger RNA export from the nucleus: a series of molecular wardrobe changes
Abstract
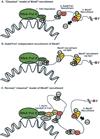
The advent of the nucleus during the evolutionary development of the eukaryotic cell necessitated the development of a transport system to convey messenger RNA (mRNA) from the site of transcription in the nucleus to ribosomes in the cytoplasm. In this review, we highlight components of each step in mRNA biogenesis, from transcription to processing, that are coupled with mRNA export from the nucleus. We also review the mechanism by which proteins from one step in the mRNA assembly line are replaced by those required for the next. These 'molecular wardrobe changes' appear to be key steps in facilitating the rapid and efficient nuclear export of mRNA transcripts.
Figures

References
-
- Moore MJ. From birth to death: the complex lives of eukaryotic mRNAs. Science. 2005;309:1514–1518. - PubMed
-
- Kang Y, Bogerd HP, Yang J, Cullen BR. Analysis of the RNA binding specificity of the human tap protein, a constitutive transport element-specific nuclear RNA export factor. Virology. 1999;262:200–209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

