Beta-catenin controls differentiation of the retinal pigment epithelium in the mouse optic cup by regulating Mitf and Otx2 expression
- PMID: 19553286
- PMCID: PMC2709060
- DOI: 10.1242/dev.032136
Beta-catenin controls differentiation of the retinal pigment epithelium in the mouse optic cup by regulating Mitf and Otx2 expression
Abstract
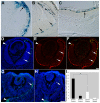
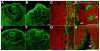
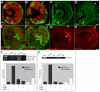
The retinal pigment epithelium (RPE) consists of a monolayer of cuboidal, pigmented cells that is located between the retina and the choroid. The RPE is vital for growth and function of the vertebrate eye and improper development results in congenital defects, such as microphthalmia or anophthalmia, or a change of cell fate into neural retina called transdifferentiation. The transcription factors microphthalmia-associated transcription factor (Mitf) and orthodenticle homolog 2 (Otx2) are crucial for RPE development and function; however, very little is known about their regulation. Here, by using a Wnt-responsive reporter, we show that the Wnt/beta-catenin pathway is activated in the differentiating mouse RPE. Cre-mediated, RPE-specific disruption of beta-catenin after the onset of RPE specification causes severe defects, resulting in microphthalmia with coloboma, disturbed lamination, and mislocalization of adherens junction proteins. Upon beta-catenin deletion, the RPE transforms into a multilayered tissue in which the expression of Mitf and Otx2 is downregulated, while retina-specific gene expression is induced, which results in the transdifferentiation of RPE into retina. Chromatin immunoprecipitation (ChIP) and luciferase assays indicate that beta-catenin binds near to and activates potential TCF/LEF sites in the Mitf and Otx2 enhancers. We conclude that Wnt/beta-catenin signaling is required for differentiation of the RPE by directly regulating the expression of Mitf and Otx2. Our study is the first to show that an extracellular signaling pathway directly regulates the expression of RPE-specific genes such as Mitf and Otx2, and elucidates a new role for the Wnt/beta-catenin pathway in organ formation and development.
Figures

References
-
- Brault, V., Moore, R., Kutsch, S., Ishibashi, M., Rowitch, D. H., McMahon, A. P., Sommer, L., Boussadia, O. and Kemler, R. (2001). Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128, 1253-1264. - PubMed
-
- Bumsted, K. M. and Barnstable, C. J. (2000). Dorsal retinal pigment epithelium differentiates as neural retina in the microphthalmia (mi/mi) mouse. Invest. Ophthalmol. Vis. Sci. 41, 903-908. - PubMed
-
- Burmeister, M., Novak, J., Liang, M. Y., Basu, S., Ploder, L., Hawes, N. L., Vidgen, D., Hoover, F., Goldman, D., Kalnins, V. I. et al. (1996). Ocular retardation mouse caused by Chx10 homeobox null allele: impaired retinal progenitor proliferation and bipolar cell differentiation. Nat. Genet. 12, 376-384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

