Palmitoylation of the influenza A virus M2 protein is not required for virus replication in vitro but contributes to virus virulence
- PMID: 19553312
- PMCID: PMC2738213
- DOI: 10.1128/JVI.01129-09
Palmitoylation of the influenza A virus M2 protein is not required for virus replication in vitro but contributes to virus virulence
Abstract
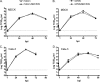
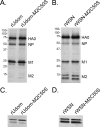
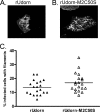
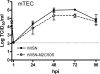
The influenza A virus M2 protein has important roles during virus entry and in the assembly of infectious virus particles. The cytoplasmic tail of the protein can be palmitoylated at a cysteine residue, but this residue is not conserved in a number of human influenza A virus isolates. Recombinant viruses encoding M2 proteins with a serine substituted for the cysteine at position 50 were generated in the A/WSN/33 (H1N1) and A/Udorn/72 (H3N2) genetic backgrounds. The recombinant viruses were not attenuated for replication in MDCK cells, Calu-3 cells, or in primary differentiated murine trachea epithelial cell cultures, indicating there was no significant contribution of M2 palmitoylation to virus replication in vitro. The A/WSN/33 M2C50S virus displayed a slightly reduced virulence after infection of mice, suggesting that there may be novel functions for M2 palmitoylation during in vivo infection.
Figures
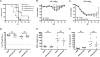
References
-
- Benton, K. A., J. A. Misplon, C. Y. Lo, R. R. Brutkiewicz, S. A. Prasad, and S. L. Epstein. 2001. Heterosubtypic immunity to influenza A virus in mice lacking IgA, all Ig, NKT cells, or gamma delta T cells. J. Immunol. 1667437-7445. - PubMed
-
- Castrucci, M. R., M. Hughes, L. Calzoletti, I. Donatelli, K. Wells, A. Takada, and Y. Kawaoka. 1997. The cysteine residues of the M2 protein are not required for influenza A virus replication. Virology 238128-134. - PubMed
-
- Centers for Disease Control and Prevention. 2009. Swine influenza A (H1N1) infection in two children—Southern California, March-April 2009. MMWR Morb. Mortal. Wkly. Rep. 58400-402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

