Kaposi's sarcoma-associated herpesvirus protein LANA2 disrupts PML oncogenic domains and inhibits PML-mediated transcriptional repression of the survivin gene
- PMID: 19553342
- PMCID: PMC2738140
- DOI: 10.1128/JVI.00339-09
Kaposi's sarcoma-associated herpesvirus protein LANA2 disrupts PML oncogenic domains and inhibits PML-mediated transcriptional repression of the survivin gene
Abstract
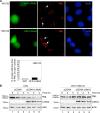
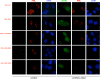
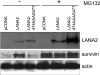
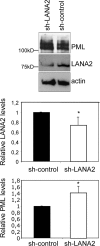
Infection by herpesviruses causes a dramatic disturbance of PML oncogenic domains (PODs) that has been suggested to be essential for viral lytic replication. Several proteins from Kaposi's sarcoma-associated herpesvirus (KSHV) have been tested as putative POD-disrupting factors with negative results. Here, we show that LANA2, a viral protein that is absolutely required for the viability and proliferation of KSHV-infected primary effusion lymphoma (PEL) cells, increases the levels of SUMO2-ubiquitin-modified PML and induces the disruption of PODs by a proteasome-mediated mechanism. In addition, we demonstrate that this disruption is largely dependent on both the integrity of a SUMO interaction motif in LANA2 and the lysine 160 from PML. Moreover, silencing of LANA2 expression in PEL cells by RNA interference led to an increase in the PML levels. Finally, we demonstrate that LANA2 relieves PML-mediated transcriptional repression of survivin, a protein that directly contributes to malignant progression of PEL. This represents the first example of inactivation of these important antiviral structures by KSHV.
Figures



References
-
- Ahn, J. H., and G. S. Hayward. 2000. Disruption of PML-associated nuclear bodies by IE1 correlates with efficient early stages of viral gene expression and DNA replication in human cytomegalovirus infection. Virology 27439-55. - PubMed
-
- Ambrosini, G., C. Adida, and D. C. Altieri. 1997. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat. Med. 3917-921. - PubMed
-
- Aoki, Y., G. M. Feldman, and G. Tosato. 2003. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood 1011535-1542. - PubMed
-
- Bernardi, R., and P. P. Pandolfi. 2007. Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nat. Rev. Mol. Cell Biol. 81006-1016. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

