Impact of serotonin 2C receptor null mutation on physiology and behavior associated with nigrostriatal dopamine pathway function
- PMID: 19553455
- PMCID: PMC3077993
- DOI: 10.1523/JNEUROSCI.3905-08.2009
Impact of serotonin 2C receptor null mutation on physiology and behavior associated with nigrostriatal dopamine pathway function
Abstract
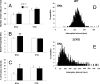
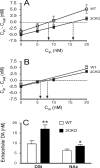
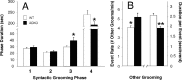
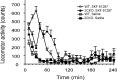
The impact of serotonergic neurotransmission on brain dopaminergic pathways has substantial relevance to many neuropsychiatric disorders. A particularly prominent role has been ascribed to the inhibitory effects of serotonin 2C receptor (5-HT(2C)R) activation on physiology and behavior mediated by the mesolimbic dopaminergic pathway, particularly in the terminal region of the nucleus accumbens. The influence of this receptor subtype on functions mediated by the nigrostriatal dopaminergic pathway is less clear. Here we report that a null mutation eliminating expression of 5-HT(2C)Rs produces marked alterations in the activity and functional output of this pathway. 5-HT(2C)R mutant mice displayed increased activity of substantia nigra pars compacta (SNc) dopaminergic neurons, elevated baseline extracellular dopamine concentrations in the dorsal striatum (DSt), alterations in grooming behavior, and enhanced sensitivity to the stereotypic behavioral effects of d-amphetamine and GBR 12909. These psychostimulant responses occurred in the absence of phenotypic differences in drug-induced extracellular dopamine concentration, suggesting a phenotypic alteration in behavioral responses to released dopamine. This was further suggested by enhanced behavioral responses of mutant mice to the D(1) receptor agonist SKF 81297. Differences in DSt D(1) or D(2) receptor expression were not found, nor were differences in medium spiny neuron firing patterns or intrinsic membrane properties following dopamine stimulation. We conclude that 5-HT(2C)Rs regulate nigrostriatal dopaminergic activity and function both at SNc dopaminergic neurons and at a locus downstream of the DSt.
Figures



References
-
- Aldridge JW, Berridge KC, Rosen AR. Basal ganglia neural mechanisms of natural movement sequences. Can J Physiol Pharmacol. 2004;82:732–739. - PubMed
-
- Alex KD, Yavanian GJ, McFarlane HG, Pluto CP, Pehek EA. Modulation of dopamine release by striatal 5-HT2C receptors. Synapse. 2005;55:242–251. - PubMed
-
- Berridge KC, Aldridge JW. Super-stereotypy II: enhancement of a complex movement sequence by intraventricular dopamine D1 agonists. Synapse. 2000;37:205–215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
