Functional significance of nonspatial information in monkey lateral intraparietal area
- PMID: 19553456
- PMCID: PMC6666030
- DOI: 10.1523/JNEUROSCI.0243-09.2009
Functional significance of nonspatial information in monkey lateral intraparietal area
Abstract
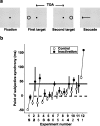
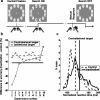
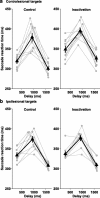
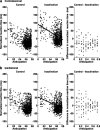
Although the parietal cortex is traditionally associated with spatial perception and motor planning, recent evidence shows that neurons in the lateral intraparietal area (LIP) carry both spatial and nonspatial signals. The functional significance of the nonspatial information in the parietal cortex is not understood. To address this question, we tested the effect of unilateral reversible inactivation of LIP on three behavioral tasks known to evoke nonspatial responses. Each task included a spatial component (target selection in the hemifield contralateral or ipsilateral to the inactivation) and a nonspatial component, namely limb motor planning, the estimation of elapsed time, and reward-based decisions. Although inactivation reliably impaired performance on all tasks, the deficits were spatially specific (restricted to contralateral target locations), and there were no effects on nonspatial aspects on performance. This suggests that modulatory nonspatial signals in LIP represent feedback about computations performed elsewhere rather than a primary role of LIP in these computations.
Figures


References
-
- Barash S, Bracewell RM, Fogassi L, Gnadt JW, Andersen RA. Saccade-related activity in the lateral intraparietal area. I. Temporal properties. J Neurophysiol. 1991;66:1095–1108. - PubMed
-
- Bisley JW, Goldberg ME. Neuronal activity in the lateral intraparietal area and spatial attention. Science. 2003;299:81–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
