Opioids depress cortical centers responsible for the volitional control of respiration
- PMID: 19553457
- PMCID: PMC6666048
- DOI: 10.1523/JNEUROSCI.1375-09.2009
Opioids depress cortical centers responsible for the volitional control of respiration
Abstract
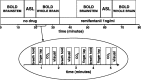
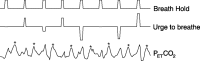
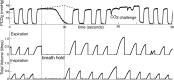
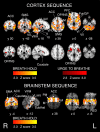
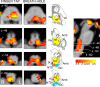
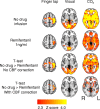
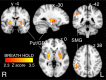
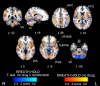
Respiratory depression limits provision of safe opioid analgesia and is the main cause of death in drug addicts. Although opioids are known to inhibit brainstem respiratory activity, their effects on cortical areas that mediate respiration are less well understood. Here, functional magnetic resonance imaging was used to examine how brainstem and cortical activity related to a short breath hold is modulated by the opioid remifentanil. We hypothesized that remifentanil would differentially depress brain areas that mediate sensory-affective components of respiration over those that mediate volitional motor control. Quantitative measures of cerebral blood flow were used to control for hypercapnia-induced changes in blood oxygen level-dependent (BOLD) signal. Awareness of respiration, reflected by an urge-to-breathe score, was profoundly reduced with remifentanil. Urge to breathe was associated with activity in the bilateral insula, frontal operculum, and secondary somatosensory cortex. Localized remifentanil-induced decreases in breath hold-related activity were observed in the left anterior insula and operculum. We also observed remifentanil-induced decreases in the BOLD response to breath holding in the left dorsolateral prefrontal cortex, anterior cingulate, the cerebellum, and periaqueductal gray, brain areas that mediate task performance. Activity in areas mediating motor control (putamen, motor cortex) and sensory-motor integration (supramarginal gyrus) were unaffected by remifentanil. Breath hold-related activity was observed in the medulla. These findings highlight the importance of higher cortical centers in providing contextual awareness of respiration that leads to appropriate modulation of respiratory control. Opioids have profound effects on the cortical centers that control breathing, which potentiates their actions in the brainstem.
Figures
References
-
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. - PubMed
-
- Andersson J, Jenkinson M, Smith SM. Oxford: Oxford Centre for Functional Magnetic Resonance Imaging of the Brain; 2007. Non-linear registration, aka spatial normalisation. FMRIB technical report TR07JA2.
-
- ATS. Dyspnea. Mechanisms, assessment, and management: a consensus statement. American Thoracic Society. Am J Respir Crit Care Med. 1999;159:321–340. - PubMed
-
- Bailey PL, Lu JK, Pace NL, Orr JA, White JL, Hamber EA, Slawson MH, Crouch DJ, Rollins DE. Effects of intrathecal morphine on the ventilatory response to hypoxia. N Engl J Med. 2000;343:1228–1234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
