The influence of neuroactive steroid lipophilicity on GABAA receptor modulation: evidence for a low-affinity interaction
- PMID: 19553485
- PMCID: PMC2724350
- DOI: 10.1152/jn.00346.2009
The influence of neuroactive steroid lipophilicity on GABAA receptor modulation: evidence for a low-affinity interaction
Abstract
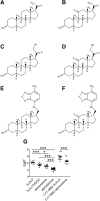
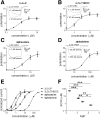
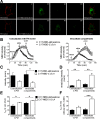
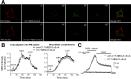
Anesthetic steroids with actions at gamma-aminobutyric acid type A receptors (GABA(A)Rs) may access transmembrane domain binding site(s) directly from the plasma cell membrane. Accordingly, the effective concentration in lipid phase and the ability of the steroid to meet pharmacophore requirements for activity will both contribute to observed steady-state potency. Furthermore, onset and offset of receptor effects may be rate limited by lipid partitioning. Here we show that several GABA-active steroids, including naturally occurring neurosteroids, of different lipophilicity differ in kinetics and potency at GABA(A)Rs. The hydrophobicity ranking predicted relative potency of GABA(A)R potentiation and predicted current offset kinetics. Kinetic offset differences among steroids were largely eliminated by gamma-cyclodextrin, a scavenger of unbound steroid, suggesting that affinity differences among the analogues are dwarfed by the contributions of nonspecific accumulation. A 7-nitrobenz-2-oxa-1,3-diazole (NBD)-tagged fluorescent analogue of the low-lipophilicity alphaxalone (C17-NBD-alphaxalone) exhibited faster nonspecific accumulation and departitioning than those of a fluorescent analogue of the high-lipophilicity (3alpha,5alpha)-3-hydroxypregnan-20-one (C17-NBD-3alpha5alphaA). These differences were paralleled by differences in potentiation of GABA(A)R function. The enantiomer of C17-NBD-3alpha5alphaA, which does not satisfy pharmacophore requirements for steroid potentiation, exhibited identical fluorescence kinetics and distribution to C17-NBD-3alpha5alphaA, but was inactive at GABA(A)Rs. Simple simulations supported our major findings, which suggest that neurosteroid binding affinity is low. Therefore both specific (e.g., fulfilling pharmacophore requirements) and nonspecific (e.g., lipid solubility) properties contribute to the potency and longevity of anesthetic steroid action.
Figures





Similar articles
-
Cyclodextrins sequester neuroactive steroids and differentiate mechanisms that rate limit steroid actions.Br J Pharmacol. 2007 Jan;150(2):164-75. doi: 10.1038/sj.bjp.0706973. Epub 2006 Dec 11. Br J Pharmacol. 2007. PMID: 17160009 Free PMC article.
-
Modulation of GABA(A) receptor function by neuroactive steroids: evidence for heterogeneity of steroid sensitivity of recombinant GABA(A) receptor isoforms.Can J Physiol Pharmacol. 1998 Sep;76(9):909-20. doi: 10.1139/cjpp-76-9-909. Can J Physiol Pharmacol. 1998. PMID: 10066142
-
The influence of the membrane on neurosteroid actions at GABA(A) receptors.Psychoneuroendocrinology. 2009 Dec;34 Suppl 1:S59-66. doi: 10.1016/j.psyneuen.2009.05.020. Psychoneuroendocrinology. 2009. PMID: 19541427 Free PMC article. Review.
-
Anticonvulsant and anesthetic effects of a fluorescent neurosteroid analog activated by visible light.Nat Neurosci. 2007 Apr;10(4):523-30. doi: 10.1038/nn1862. Epub 2007 Feb 25. Nat Neurosci. 2007. PMID: 17322875
-
Plasticity and function of extrasynaptic GABA(A) receptors during pregnancy and after delivery.Psychoneuroendocrinology. 2009 Dec;34 Suppl 1:S74-83. doi: 10.1016/j.psyneuen.2009.06.013. Psychoneuroendocrinology. 2009. PMID: 19608348 Review.
Cited by
-
Neuroendocrine aspects of catamenial epilepsy.Horm Behav. 2013 Feb;63(2):254-66. doi: 10.1016/j.yhbeh.2012.04.016. Epub 2012 May 2. Horm Behav. 2013. PMID: 22579656 Free PMC article. Review.
-
Neurosteroid Structure-Activity Relationships for Functional Activation of Extrasynaptic δGABA(A) Receptors.J Pharmacol Exp Ther. 2016 Apr;357(1):188-204. doi: 10.1124/jpet.115.229302. Epub 2016 Feb 8. J Pharmacol Exp Ther. 2016. PMID: 26857959 Free PMC article.
-
A clickable neurosteroid photolabel reveals selective Golgi compartmentalization with preferential impact on proximal inhibition.Neuropharmacology. 2016 Sep;108:193-206. doi: 10.1016/j.neuropharm.2016.04.031. Epub 2016 Apr 22. Neuropharmacology. 2016. PMID: 27114255 Free PMC article.
-
Neurosteroid Modulation of GABAA Receptor Function by Independent Action at Multiple Specific Binding Sites.Curr Neuropharmacol. 2022;20(5):886-890. doi: 10.2174/1570159X19666211202150041. Curr Neuropharmacol. 2022. PMID: 34856904 Free PMC article.
-
Characteristics of concatemeric GABA(A) receptors containing α4/δ subunits expressed in Xenopus oocytes.Br J Pharmacol. 2012 Apr;165(7):2228-43. doi: 10.1111/j.1476-5381.2011.01690.x. Br J Pharmacol. 2012. PMID: 21950777 Free PMC article.
References
-
- Adam JM, Bennett DJ, Bom A, Clark JK, Feilden H, Hutchinson EJ, Palin R, Prosser A, Rees DC, Rosair GM, Stevenson D, Tarver GJ, Zhang MQ. Cyclodextrin-derived host molecules as reversal agents for the neuromuscular blocker rocuronium bromide: synthesis and structure–activity relationships. J Med Chem 45: 1806–1816, 2002. - PubMed
-
- Alakoskela JM, Covey DF, Kinnunen PK. Lack of enantiomeric specificity in the effects of anesthetic steroids on lipid bilayers. Biochim Biophys Acta 1768: 131–145, 2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

