Trans-presentation of IL-15 by intestinal epithelial cells drives development of CD8alphaalpha IELs
- PMID: 19553528
- PMCID: PMC2706935
- DOI: 10.4049/jimmunol.0900420
Trans-presentation of IL-15 by intestinal epithelial cells drives development of CD8alphaalpha IELs
Abstract
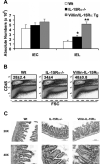
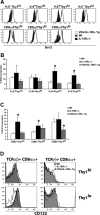
IL-15 is crucial for the development of intestinal intraepithelial lymphocytes (IEL) and delivery is mediated by a unique mechanism known as trans-presentation. Parenchymal cells have a major role in the trans-presentation of IL-15 to IELs, but the specific identity of this cell type is unknown. To investigate whether the intestinal epithelial cells (IEC) are the parenchymal cell type involved, a mouse model that expresses IL-15Ralpha exclusively by the IECs (Villin/IL-15Ralpha Tg) was generated. Exclusive expression of IL-15Ralpha by the IECs restored all the deficiencies in the CD8alphaalpha(+)TCRalphabeta(+)and CD8alphaalpha(+)TCRgammadelta(+) subsets that exist in the absence of IL-15Ralpha. Interestingly, most of the IEL recovery was due to the preferential increase in Thy1(low) IELs, which compose a majority of the IEL population. The differentiation of Thy1(high)CD4(-)CD8(-) thymocytes into Thy1(-)CD8alphaalpha IELs was found to require IL-15Ralpha expression specifically by IECs and thus, provides evidence that differentiation of Thy1(low) IELs is one function of trans-presentation of IL-15 in the intestines. In addition to effects in IEL differentiation, trans-presentation of IL-15 by IECs also resulted in an increase in IEL numbers that was accompanied by increases in Bcl-2, but not proliferation. Collectively, this study demonstrates that trans-presentation of IL-15 by IECs alone is completely sufficient to direct the IL-15-mediated development of CD8alphaalpha(+) T cell populations within the IEL compartment, which now includes a newly identified role of IL-15 in the differentiation of Thy1(low) IELs.
Figures



References
-
- Lefrancois L. Phenotypic complexity of intraepithelial lymphocytes of the small intestine. J Immunol. 1991;147:1746–1751. - PubMed
-
- Kim SK, Schluns KS, Lefrancois L. Induction and visualization of mucosal memory CD8 T cells following systemic virus infection. Journal of Immunology. 1999;163:4125–4132. - PubMed
-
- Jarry A, Cerf-Bensussan N, Brousse N, Selz F, Guy-Grand D. Subsets of CD3+ (T cell receptor alpha/beta or gamma/delta) and CD3- lymphocytes isolated from normal human gut epithelium display phenotypical features different from their counterparts in peripheral blood. Eur. J. Immunol. 1990;20:1097–1103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

