Development and technical and clinical validation of a quantitative enzyme-linked immunosorbent assay for the detection of human antibodies to hepatitis B surface antigen in recipients of recombinant hepatitis B virus vaccine
- PMID: 19553553
- PMCID: PMC2725549
- DOI: 10.1128/CVI.00431-08
Development and technical and clinical validation of a quantitative enzyme-linked immunosorbent assay for the detection of human antibodies to hepatitis B surface antigen in recipients of recombinant hepatitis B virus vaccine
Abstract
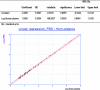
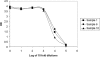
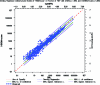
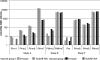
Pending removal from the market of a commercial assay (the AUSAB [Abbott Laboratories] enzyme immunoassay [EIA]) for the determination of antibodies to hepatitis B surface antigen (HBsAg), a new in-house quantitative enzyme-linked immunosorbent assay (ELISA) to measure antibodies against HBsAg (anti-HBs) was developed (anti-HBs in-house). Specific anti-HBs antibodies were sandwiched between the precoated HBsAg ad and ay subtypes purified from plasma from hepatitis B virus (HBV) human carriers and the recombinant HBsAg adw2 subtype tagged with horseradish peroxidase. The assay was calibrated against the 1st International Reference Preparation for anti-hepatitis B immunoglobulin (lot 1977-W1042). Analytical sensitivity and the limit of quantitation were estimated at 0.43 mIU/ml and 2.0 mIU/ml, respectively. Overall reproducibility was 11.86%, and accuracy was estimated to be 94.89%. More than 4,000 samples from seven clinical trials were tested with the anti-HBs in-house assay and compared to results generated with AUSAB EIA and AUSAB radioimmunoassay (RIA). During the technical validation, the anti-HBs in-house assay was compared to the AUSAB RIA as a reference (n = 919). Overall assessment of concordance and Deming's regression analysis were performed. The coefficient of correlation between the AUSAB RIA and anti-HBs in-house assay was 0.9815 with a slope of 0.9187. The overall agreement between anti-HBs in-house and AUSAB RIA was 97.61%, considering the clinical cutoffs at 3.3 mIU/ml and 1.0 mIU/ml for the respective assays. From a clinical perspective, seroprotection rates and anti-HBs geometric mean antibody concentrations for individual studies calculated with either the in-house assay or the reference assays were similar. Conclusions of individual studies were confirmed. The performance characteristics of the in-house assay are acceptable. There is no evidence that use of the new assay would lead to different clinical conclusions from the reference method.
Figures

References
-
- Barker, L. F., D. Lorenz, S. C. Rastogi, J. S. Finlayson, and E. B. Seligmann. 1977. Study of a proposed international reference preparation for anti-hepatitis B immunoglobulin. Expert Committee on Biological Standardization technical report series. World Health Organization, Geneva Switzerland.
-
- Braggio, S., R. J. Barnaby, P. Grossi, and M. Cugola. 1996. A strategy for validation of bioanalytical methods. J. Pharm. Biomed. Anal. 14375-388. - PubMed
-
- Findlay, J. W. A., W. C. Smith, J. W. Lee, G. D. Nordblom, I. Das, B. S. DeSilva, M. N. Khan, and R. R. Bowsher. 2000. Validation of immunoassays for bioanalysis: a pharmaceutical industry perspective. J. Pharm. Biomed. Anal. 211249-1273. - PubMed
-
- Hauser, P., P. Voet, E. Simoen, H. C. Thomas, J. Pêtre, M. De Wilde, and J. Stephenne. 1987. Immunological properties of recombinant HBsAg produced in yeast. Postgrad. Med. J. 6383-91. - PubMed
-
- Keating, G. M., and S. Noble. 2003. Recombinant hepatitis B vaccine (Engerix™-B): a review of its immunogenicity and protective efficacy against hepatitis B. Drugs 631021-1051. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

