Proteomic identification of OprL as a seromarker for initial diagnosis of Pseudomonas aeruginosa infection of patients with cystic fibrosis
- PMID: 19553571
- PMCID: PMC2725635
- DOI: 10.1128/JCM.02182-08
Proteomic identification of OprL as a seromarker for initial diagnosis of Pseudomonas aeruginosa infection of patients with cystic fibrosis
Abstract
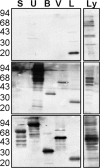
Identification of new immunogenic antigens that diagnose initial Pseudomonas aeruginosa infections in patients with cystic fibrosis (CF) alone or as an adjunct to microbiology is needed. In the present study, a proteomic analysis was performed to obtain a global assessment of the host immune response during the initial P. aeruginosa infection of patients with CF. Matrix-assisted laser desorption ionization-time of flight mass spectrometry was used to identify outer membrane protein L (OprL), a non-type III secretion system (TTSS) protein, as an early immunogenic protein during the initial P. aeruginosa infection of patients with CF. Longitudinal Western blot analysis of sera from 12 of 14 patients with CF detected antibodies to OprL during the initial P. aeruginosa infection. In addition, also detected were antibodies to ExoS, ExoU, or ExoS and ExoU, the latter indicating sequential P. aeruginosa infections during initial infections. Detection of serum reactivity to OprL, along with proteins of the TTSS, and in conjunction with microbiology may diagnose initial P. aeruginosa infections in patients with CF.
Figures


References
-
- Aebi, C., R. Bracher, S. Liechti-Gallati, H. Tschappeler, A. Rudeberg, and R. Kraemer. 1995. The age at onset of chronic Pseudomonas aeruginosa colonization in cystic fibrosis—prognostic significance. Eur. J. Pediatr. 154S69-S73. - PubMed
-
- Baumann, U., C. Stocklossa, W. Greiner, J. M. von der Schulenburg, and H. von der Hardt. 2003. Cost of care and clinical condition in paediatric cystic fibrosis patients. J. Cyst. Fibros. 284-90. - PubMed
-
- Burns, J. L., R. L. Gibson, S. McNamara, D. Yim, J. Emerson, M. Rosenfeld, P. Hiatt, K. McCoy, R. Castile, A. L. Smith, and B. W. Ramsey. 2001. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J. Infect. Dis. 183444-452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

