Linker histones stimulate HSPA2 ATPase activity through NASP binding and inhibit CDC2/Cyclin B1 complex formation during meiosis in the mouse
- PMID: 19553603
- PMCID: PMC2754887
- DOI: 10.1095/biolreprod.109.076497
Linker histones stimulate HSPA2 ATPase activity through NASP binding and inhibit CDC2/Cyclin B1 complex formation during meiosis in the mouse
Abstract
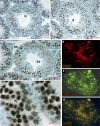
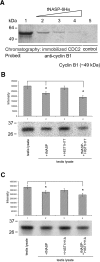
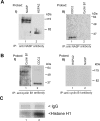
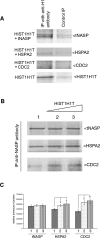
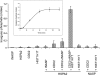
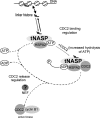
In mammalian spermatocytes, cell division cycle protein 2 (CDC2)/cyclin B1 and the chaperone heat shock protein A2 (HSPA2) are required for the G2-->M transition in prophase I. Here, we demonstrate that in primary spermatocytes, linker histone chaperone testis/embryo form of nuclear autoantigenic sperm protein (tNASP) binds the heat shock protein HSPA2, which localizes on the synaptonemal complex of spermatocytes. Significantly, the tNASP-HSPA2 complex binds linker histones and CDC2, forming a larger complex. We demonstrate that increasing amounts of tNASP favor tNASP-HSPA2-CDC2 complex formation. Binding of linker histones to tNASP significantly increases HSPA2 ATPase activity and the capacity of tNASP to bind HSPA2 and CDC2, precluding CDC2/cyclin B1 complex formation and, consequently, decreasing CDC2/cyclin B1 kinase activity. Linker histone binding to NASP controls the ability of HSPA2 to activate CDC2 for CDC2/cyclin B1 complex formation; therefore, tNASP's role is to provide the functional link between linker histones and cell cycle progression during meiosis.
Figures

References
-
- Govin J, Caron C, Lestrat C, Rousseaux S, Khochbin S.The role of histones in chromatin remodelling during mammalian spermiogenesis. Eur J Biochem 2004; 271: 3459–3469.. - PubMed
-
- van der Heijden GW, Derijck AA, Pósfai E, Giele M, Pelczar P, Ramos L, Wansink DG, van der Vlag J, Peters AH, de Boer P.Chromosome-wide nucleosome replacement and H3.3 incorporation during mammalian meiotic sex chromosome inactivation. Nat Genet 2007; 39: 251–258.. - PubMed
-
- Groth A, Rocha W, Verreault A, Almouzni G.Chromatin challenges during DNA replication and repair. Cell 2007; 128: 721–733.. - PubMed
-
- Hochwagen A, Amon A.Checking your breaks: surveillance mechanisms of meiotic recombination. Curr Biol 2006; 16: R217–R228.. - PubMed
-
- Pérez-Hidalgo L, Moreno S, San-Segundo PA.Regulation of meiotic progression by the meiosis-specific checkpoint kinase Mek1 in fission yeast. J Cell Sci 2003; 116: 259–271.. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

