Limited roles of Rdh8, Rdh12, and Abca4 in all-trans-retinal clearance in mouse retina
- PMID: 19553623
- PMCID: PMC2792889
- DOI: 10.1167/iovs.09-3944
Limited roles of Rdh8, Rdh12, and Abca4 in all-trans-retinal clearance in mouse retina
Abstract
Purpose: Although the retinoid cycle is essential for vision, all-trans-retinal and the side products of this cycle are toxic. Delayed clearance of all-trans-retinal causes accumulation of its condensation products, A2E, and all-trans-retinal dimer (RALdi), both associated with human macular degeneration. The protective roles were examined of the all-trans-RDHs, Rdh8 and Rdh12, and the ATP-binding cassette transporter Abca4, retinoid cycle enzymes involved in all-trans-retinal clearance.
Methods: Mice genetically engineered to lack Rdh8, Rdh12, and Abca4, either singly or in various combinations, were investigated because all-trans-retinal clearance is achieved by all-trans-RDHs and Abca4. Knockout mice were evaluated by spectral-domain optical coherence tomography (SD-OCT), electroretinography, retinal morphology, and visual retinoid profiling with HPLC and MS. ARPE19 cells were examined to evaluate A2E and RALdi oxidation and toxicity induced by exposure to UV and blue light.
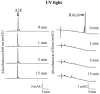
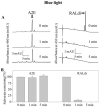
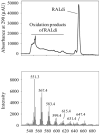
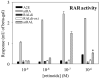
Results: Rdh8(-/-)Abca4(-/-) and Rdh8(-/-)Rdh12(-/-)Abca4(-/-) mice displayed slowly progressive, severe retinal degeneration under room light conditions. Intense light-induced acute retinal degeneration was detected by SD-OCT in Rdh8(-/-)Rdh12(-/-)Abca4(-/-) mice. Amounts of A2E in the RPE correlated with diminished all-trans-retinal clearance, and the highest A2E amounts were found in Rdh8(-/-)Rdh12(-/-)Abca4(-/-) mice. However oxidized A2E was not found in any of these mice, and A2E oxidation was not induced by blue light and UV illumination of A2E-loaded ARPE19 cells. Of interest, addition of all-trans-retinal did activate retinoic acid receptors in cultured cells.
Conclusions: Rdh8, Rdh12, and Abca4 all protect the retina and reduce A2E production by facilitating all-trans-retinal clearance. Delayed all-trans-retinal clearance contributes more than A2E oxidation to light-induced cellular toxicity.
Conflict of interest statement
Disclosure:
Figures

Similar articles
-
Redundant and unique roles of retinol dehydrogenases in the mouse retina.Proc Natl Acad Sci U S A. 2007 Dec 4;104(49):19565-70. doi: 10.1073/pnas.0707477104. Epub 2007 Nov 28. Proc Natl Acad Sci U S A. 2007. PMID: 18048336 Free PMC article.
-
Evaluation of potential therapies for a mouse model of human age-related macular degeneration caused by delayed all-trans-retinal clearance.Invest Ophthalmol Vis Sci. 2009 Oct;50(10):4917-25. doi: 10.1167/iovs.09-3581. Epub 2009 Jun 3. Invest Ophthalmol Vis Sci. 2009. PMID: 19494204 Free PMC article.
-
Exposure of A2E to blue light promotes ferroptosis in the retinal pigment epithelium.Cell Mol Biol Lett. 2025 Feb 21;30(1):22. doi: 10.1186/s11658-025-00700-2. Cell Mol Biol Lett. 2025. PMID: 39984833 Free PMC article.
-
Retinol dehydrogenases (RDHs) in the visual cycle.Exp Eye Res. 2010 Dec;91(6):788-92. doi: 10.1016/j.exer.2010.08.013. Epub 2010 Aug 27. Exp Eye Res. 2010. PMID: 20801113 Free PMC article. Review.
-
A2E, a byproduct of the visual cycle.Vision Res. 2003 Dec;43(28):2983-90. doi: 10.1016/s0042-6989(03)00475-9. Vision Res. 2003. PMID: 14611934 Review.
Cited by
-
Photoreceptor proteins initiate microglial activation via Toll-like receptor 4 in retinal degeneration mediated by all-trans-retinal.J Biol Chem. 2013 May 24;288(21):15326-41. doi: 10.1074/jbc.M112.448712. Epub 2013 Apr 9. J Biol Chem. 2013. PMID: 23572532 Free PMC article.
-
Optical coherence tomography: history, current status, and laboratory work.Invest Ophthalmol Vis Sci. 2011 Apr 14;52(5):2425-36. doi: 10.1167/iovs.10-6312. Print 2011 Apr. Invest Ophthalmol Vis Sci. 2011. PMID: 21493951 Free PMC article. Review.
-
Phenotypic high-throughput screening identifies aryl hydrocarbon receptor agonism as common inhibitor of toxin-induced retinal pigment epithelium cell death.PLoS One. 2024 Apr 18;19(4):e0301239. doi: 10.1371/journal.pone.0301239. eCollection 2024. PLoS One. 2024. PMID: 38635505 Free PMC article.
-
Vitamin A activates rhodopsin and sensitizes it to ultraviolet light.Vis Neurosci. 2011 Nov;28(6):485-97. doi: 10.1017/S0952523811000423. Vis Neurosci. 2011. PMID: 22192505 Free PMC article.
-
Quercetin and cyanidin-3-glucoside protect against photooxidation and photodegradation of A2E in retinal pigment epithelial cells.Exp Eye Res. 2017 Jul;160:45-55. doi: 10.1016/j.exer.2017.04.010. Epub 2017 Apr 28. Exp Eye Res. 2017. PMID: 28461203 Free PMC article.
References
-
- Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt's disease from the phenotype in abcr knockout mice. Cell. 1999;98:13–23. - PubMed
-
- Rattner A, Smallwood PM, Nathans J. Identification and characterization of all-trans-retinol dehydrogenase from photoreceptor outer segments, the visual cycle enzyme that reduces all-trans-retinal to all-trans-retinol. J Biol Chem. 2000;275:11034–11043. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

