Inhibition of chemokine receptor expression on uveal melanomas by CXCR4 siRNA and its effect on uveal melanoma liver metastases
- PMID: 19553629
- PMCID: PMC2841954
- DOI: 10.1167/iovs.09-3804
Inhibition of chemokine receptor expression on uveal melanomas by CXCR4 siRNA and its effect on uveal melanoma liver metastases
Abstract
Purpose: To determine whether blocking the expression of the chemokine receptor CXCR4 using siRNA inhibits chemotactic responses of human uveal melanoma cells to liver-derived factors and prevents liver metastases.
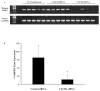
Methods: Human uveal melanoma cells were transfected with CXCR4 siRNA or control siRNA and tested in vitro for chemotactic and invasive behavior in response to soluble factors produced by human liver cells. The effect of CXCR4 siRNA transfection on the formation of liver metastases was tested by injecting transfected melanoma cells into the spleen capsules of NOD-SCID mice, and metastases were quantified by measuring the human housekeeping gene hHPRT in livers.
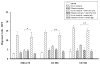
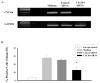
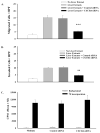
Results: Blocking CXCR4 interaction with its ligand using anti-CXCL12 antibody resulted in a significant reduction in the chemotactic responses of uveal melanoma cells to soluble factors produced by human liver cells. Similarly, blocking CXCR4 gene expression by transfection with CXCR4 siRNA inhibited both the chemotactic and the invasive properties of uveal melanoma cells exposed to factors produced by human livers. Uveal melanoma cells transfected with CXCR4 siRNA produced fewer liver metastases than untreated uveal melanoma cells or uveal melanoma cells transfected with control siRNA.
Conclusions: CXCR4 is a key chemokine receptor that may account for the organ-specific homing of human uveal melanomas to the liver, which contains significant quantities of CXCL2, the only known ligand for CXCR4. CXCR4 is a potential therapeutic target for preventing the initial establishment of liver metastases but has limited application for use in advanced liver tumors.
Figures

Similar articles
-
Differential expression of chemokine receptors on uveal melanoma cells and their metastases.Invest Ophthalmol Vis Sci. 2008 Feb;49(2):636-43. doi: 10.1167/iovs.07-1035. Invest Ophthalmol Vis Sci. 2008. PMID: 18235009
-
Chemokine receptor CCR7 expression predicts poor outcome in uveal melanoma and relates to liver metastasis whereas expression of CXCR4 is not of clinical relevance.Invest Ophthalmol Vis Sci. 2013 Nov 8;54(12):7354-61. doi: 10.1167/iovs.13-12407. Invest Ophthalmol Vis Sci. 2013. PMID: 24052640
-
Expression of haematogenous and lymphogenous chemokine receptors and their ligands on uveal melanoma in association with liver metastasis.Acta Ophthalmol. 2012 Dec;90(8):e638-44. doi: 10.1111/j.1755-3768.2012.02515.x. Epub 2012 Nov 20. Acta Ophthalmol. 2012. PMID: 23164171
-
Biologic determinants of uveal melanoma metastatic phenotype: role of intermediate filaments as predictive markers.Lab Invest. 1998 Feb;78(2):153-63. Lab Invest. 1998. PMID: 9484713 Review.
-
Molecular pathways mediating liver metastasis in patients with uveal melanoma.Clin Cancer Res. 2008 Feb 15;14(4):951-6. doi: 10.1158/1078-0432.CCR-06-2630. Clin Cancer Res. 2008. PMID: 18281525 Review.
Cited by
-
Epigenetic regulation of CXCR4 expression by the ocular microenvironment.Invest Ophthalmol Vis Sci. 2013 Jan 9;54(1):234-43. doi: 10.1167/iovs.12-10643. Invest Ophthalmol Vis Sci. 2013. PMID: 23188729 Free PMC article.
-
Chemokine receptor 4 targeted protein MRI contrast agent for early detection of liver metastases.Sci Adv. 2020 Feb 7;6(6):eaav7504. doi: 10.1126/sciadv.aav7504. eCollection 2020 Feb. Sci Adv. 2020. PMID: 32083172 Free PMC article.
-
Role of chemokines in hepatocellular carcinoma (Review).Oncol Rep. 2021 Mar;45(3):809-823. doi: 10.3892/or.2020.7906. Epub 2020 Dec 22. Oncol Rep. 2021. PMID: 33650640 Free PMC article. Review.
-
Identifying a Potential Key Gene, TIMP1, Associated with Liver Metastases of Uveal Melanoma by Weight Gene Co-Expression Network Analysis.Onco Targets Ther. 2020 Nov 19;13:11923-11934. doi: 10.2147/OTT.S280435. eCollection 2020. Onco Targets Ther. 2020. PMID: 33239893 Free PMC article.
-
Genetic evolution of uveal melanoma guides the development of an inflammatory microenvironment.Cancer Immunol Immunother. 2017 Jul;66(7):903-912. doi: 10.1007/s00262-017-1991-1. Epub 2017 Apr 8. Cancer Immunol Immunother. 2017. PMID: 28391358 Free PMC article.
References
-
- Egan KM, Seddon JM, Glynn RJ, Gragoudas ES, Albert DM. Epidemiologic aspects of uveal melanoma. Surv Ophthalmol. 1988;32:239–251. - PubMed
-
- Bedikian AY, Legha SS, Mavligit G, et al. Treatment of uveal melanoma metastatic to the liver: a review of the M. D. Anderson Cancer Center experience and prognostic factors. Cancer. 1995;76:1665–1670. - PubMed
-
- Albert DM. The ocular melanoma story: LIII Edward Jackson Memorial Lecture, part II. Am J Ophthalmol. 1997;123:729–741. - PubMed
-
- Gombos DS, Mieler WF. Therapy of uveal melanoma: methods and risk factors associated with treatment. In: Albert DM, Polans A, editors. Ocular Oncology. New York: Marcel Dekker, Inc; 2003. pp. 321–352.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

