Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements
- PMID: 19554048
- PMCID: PMC2767162
- DOI: 10.1038/nature08209
Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements
Abstract
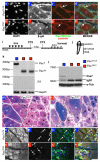
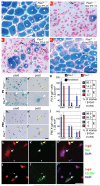
Myogenic potential, survival and expansion of mammalian muscle progenitors depend on the myogenic determinants Pax3 and Pax7 embryonically, and Pax7 alone perinatally. Several in vitro studies support the critical role of Pax7 in these functions of adult muscle stem cells (satellite cells), but a formal demonstration has been lacking in vivo. Here we show, through the application of inducible Cre/loxP lineage tracing and conditional gene inactivation to the tibialis anterior muscle regeneration paradigm, that, unexpectedly, when Pax7 is inactivated in adult mice, mutant satellite cells are not compromised in muscle regeneration, they can proliferate and reoccupy the sublaminal satellite niche, and they are able to support further regenerative processes. Dual adult inactivation of Pax3 and Pax7 also results in normal muscle regeneration. Multiple time points of gene inactivation reveal that Pax7 is only required up to the juvenile period when progenitor cells make the transition into quiescence. Furthermore, we demonstrate a cell-intrinsic difference between neonatal progenitor and adult satellite cells in their Pax7-dependency. Our finding of an age-dependent change in the genetic requirement for muscle stem cells cautions against inferring adult stem-cell biology from embryonic studies, and has direct implications for the use of stem cells from hosts of different ages in transplantation-based therapy.
Figures


Comment in
-
Developmental biology: Skeletal muscle comes of age.Nature. 2009 Jul 30;460(7255):584-5. doi: 10.1038/460584a. Nature. 2009. PMID: 19641585 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

