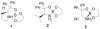Enantioselective Reduction of Prochiral Ketones using Spiroborate Esters as Catalysts
- PMID: 19554205
- PMCID: PMC2701200
- DOI: 10.1016/j.tetlet.2007.06.086
Enantioselective Reduction of Prochiral Ketones using Spiroborate Esters as Catalysts
Abstract
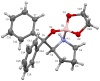
Novel spiroborate esters derived nonracemic 1,2-aminoalcohols and ethylene glycol are reported as highly effective catalysts for the asymmetric borane reduction of a variety of prochiral ketones with borane-dimethyl sulfide complex at room temperature. Optically active alcohols were obtained in excellent chemical yields using 0.1 to 10 mol % of catalysts with up to 99% ee.
Figures
References
-
- Silverman RB. The Organic Chemistry of Drug Design and Drug Action. Elsevier Academic Press; Burlington, MA: 2004.
- Challenger CA. Chiral Drugs. John Wiley & Sons; New york: 2001.
-
- Ager DJ. Chiral Chemicals. Marcel Dekker, Inc; New York: 1999.
-
- Morris DJ, Hayes AM, Wills M. J Org Chem. 2006;71:7035–7044. - PubMed
-
- Schiffers I, Rantanen T, Schmidt F, Bergmans W, Zani L, Bolm C. J Org Chem. 2006;71:2320–2331. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources