Nonlinear pharmacokinetics of therapeutic proteins resulting from receptor mediated endocytosis
- PMID: 19554432
- PMCID: PMC2718226
- DOI: 10.1007/s10928-009-9120-1
Nonlinear pharmacokinetics of therapeutic proteins resulting from receptor mediated endocytosis
Abstract
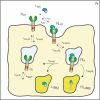
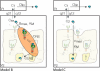
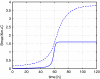
Receptor mediated endocytosis (RME) plays a major role in the disposition of therapeutic protein drugs in the body. It is suspected to be a major source of nonlinear pharmacokinetic behavior observed in clinical pharmacokinetic data. So far, mostly empirical or semi-mechanistic approaches have been used to represent RME. A thorough understanding of the impact of the properties of the drug and of the receptor system on the resulting nonlinear disposition is still missing, as is how to best represent RME in pharmacokinetic models. In this article, we present a detailed mechanistic model of RME that explicitly takes into account receptor binding and trafficking inside the cell and that is used to derive reduced models of RME which retain a mechanistic interpretation. We find that RME can be described by an extended Michaelis-Menten model that accounts for both the distribution and the elimination aspect of RME. If the amount of drug in the receptor system is negligible a standard Michaelis-Menten model is capable of describing the elimination by RME. Notably, a receptor system can efficiently eliminate drug from the extracellular space even if the total number of receptors is small. We find that drug elimination by RME can result in substantial nonlinear pharmacokinetics. The extent of nonlinearity is higher for drug/receptor systems with higher receptor availability at the membrane, or faster internalization and degradation of extracellular drug. Our approach is exemplified for the epidermal growth factor receptor system.
Figures




References
-
- Meibohm B (2006) Pharmacokinetics and pharmacodynamics of biotech drugs. Wiley-VCH Verlag, Weinheim
-
- Kuester K, Kloft C (2006) Pharmacokinetics of monoclonal antibodies. In Meibohm B (ed) Pharmacokinetics and pharmacodynamics of biotech drugs, chapter 3. Wiley-VCH Verlag, Weinheim, pp 45–91
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

