Olfactomedin domain-containing proteins: possible mechanisms of action and functions in normal development and pathology
- PMID: 19554483
- PMCID: PMC2936706
- DOI: 10.1007/s12035-009-8076-x
Olfactomedin domain-containing proteins: possible mechanisms of action and functions in normal development and pathology
Abstract
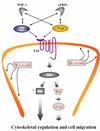
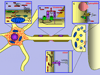
A family of olfactomedin domain-containing proteins consists of at least 13 members in mammals. Although the first protein belonging to this family, olfactomedin, was isolated and partially characterized from frog olfactory neuroepithelim almost 20 years ago, the functions of many family members remain elusive. Most of the olfactomedin domain-containing proteins, similar to frog olfactomedin, are secreted glycoproteins that demonstrate specific expression patterns. Other family members are membrane-bound proteins that may serve as receptors. More than half of the olfactomedin domain-containing genes are expressed in neural tissues. Data obtained over the last several years demonstrate that olfactomedin domain-containing proteins play important roles in neurogenesis, neural crest formation, dorsal ventral patterning, cell-cell adhesion, cell cycle regulation, and tumorigenesis and may serve as modulators of critical signaling pathways (Wnt, bone morphogenic protein). Mutations in two genes encoding myocilin and olfactomedin 2 were implicated in glaucoma, and a growing number of evidence indicate that other genes belonging to the family of olfactomedin domain-containing proteins may contribute to different human disorders including psychiatric disorders. In this review, we summarize recent advances in understanding the possible roles of these proteins with special emphasis on the proteins that are preferentially expressed and function in neural tissues.
Figures



References
-
- Snyder DA, Rivers AM, Yokoe H, Menco BP, Anholt RR. Olfactomedin: purification, characterization, and localization of a novel olfactory glycoprotein. Biochemistry. 1991;30:9143–9153. - PubMed
-
- Karavanich CA, Anholt RR. Molecular evolution of olfactomedin. Mol Biol Evol. 1998;15:718–726. - PubMed
-
- Zeng LC, Han ZG, Ma WJ. Elucidation of subfamily segregation and intramolecular coevolution of the olfactomedin-like proteins by comprehensive phylogenetic analysis and gene expression pattern assessment. FEBS Lett. 2005;579:5443–5453. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

