Soluble polymer carriers for the treatment of cancer: the importance of molecular architecture
- PMID: 19555070
- PMCID: PMC2759385
- DOI: 10.1021/ar900035f
Soluble polymer carriers for the treatment of cancer: the importance of molecular architecture
Abstract
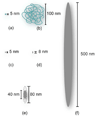
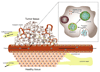
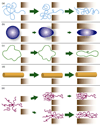
Chemotherapy can destroy tumors and arrest cancer progress. Unfortunately, severe side effects (treatment is usually a series of injections of highly toxic drugs) often restrict the frequency and size of dosages, much to the detriment of tumor inhibition. Most chemotherapeutic drugs have pharmacokinetic profiles with tremendous potential for improvement. Water-soluble polymers offer the potential to increase drug circulation time, improve drug solubility, prolong drug residence time in a tumor, and reduce toxicity. Cytotoxic drugs that are covalently attached to water-soluble polymers via reversible linkages more effectively target tumor tissue than the drugs alone. Macromolecules passively target solid tumor tissue through a combination of reduced renal clearance and exploitation of the enhanced permeation and retention (EPR) effect, which prevails for fast-growing tumors. Effective drug delivery involves a balance between (i) elimination of the polymeric drug conjugate from the bloodstream by the kidneys, liver, and other organs and (ii) movement of the drug out of the blood vasculature and into the tumor (that is, extravasation). Polymers are eliminated in the kidney by filtration through pores with a size comparable to the hydrodynamic diameter of the polymer; in contrast, the openings in the blood vessel structures that traverse tumors are an order of magnitude greater than the diameter of the polymer. Thus, features that may broadly be grouped as the "molecular architecture" of the polymer, such as its hydrodynamic volume (or molecular weight), molecular conformation, chain flexibility, branching, and location of the attached drug, can greatly impact elimination of the polymer from the body through the kidney but have a much smaller effect on the extravasation of the polymer into the tumor. Molecular architecture can in theory be adjusted to assert essentially independent control over elimination and extravasation. Understanding how molecular architecture affects passage of a polymer through a pore is therefore essential for designing polymer drug carriers that are effective in passively delivering a drug payload while conforming to the requirement that the polymers must eventually be eliminated from the body. In this Account, we discuss examples from in vivo studies that demonstrate how polymer architectural features impact the renal filtration of a polymer as well as tumor penetration and tumor accumulation. In brief, features that inhibit passage of a polymer through a pore, such as higher molecular weight, decreased flexibility, and an increased number of polymer chain ends, help prevent elimination of the polymer by the kidneys and can improve blood circulation times and tumor accumulation, thus improving therapeutic effectiveness.
Figures



References
-
- Bader H, Ringsdorf H, Schmidt B. Water-Soluble Polymers in Medicine. Angew. Makromol. Chem. 1984;123/124:457–485.
-
- Kopecek J. Soluble Biomedical Polymers. Polymers in Medicine. 1977;7:191–221. - PubMed
-
- Duncan R. The Dawning Era of Polymer Therapeutics. Nature Reviews Drug Discovery. 2003;2:347–360. - PubMed
-
- Lee CC, Mackay JA, Fréchet JMJ, Szoka FC. Designing Dendrimers for Biological Applications. Nat. Biotechnol. 2005;23:1517–1526. - PubMed
-
- Liu MJ, Fréchet JMJ. Designing Dendrimers for Drug Delivery. Pharmaceutical Science & Technology Today. 1999;2:393–401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

