Self-organization in coordination-driven self-assembly
- PMID: 19555073
- PMCID: PMC2764814
- DOI: 10.1021/ar900077c
Self-organization in coordination-driven self-assembly
Abstract
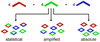
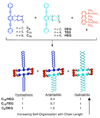
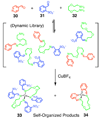
Self-assembly allows for the preparation of highly complex molecular and supramolecular systems from relatively simple starting materials. Typically, self-assembled supramolecules are constructed by combining complementary pairs of two highly symmetric molecular components, thus limiting the chances of forming unwanted side products. Combining asymmetric molecular components or multiple complementary sets of molecules in one complex mixture can produce myriad different ordered and disordered supramolecular assemblies. Alternatively, spontaneous self-organization phenomena can promote the formation of specific product(s) out of a collection of multiple possibilities. Self-organization processes are common throughout much of nature and are especially common in biological systems. Recently, researchers have studied self-organized self-assembly in purely synthetic systems. This Account describes our investigations of self-organization in the coordination-driven self-assembly of platinum(II)-based metallosupramolecules. The modularity of the coordination-driven approach to self-assembly has allowed us to systematically study a wide variety of different factors that can control the extent of supramolecular self-organization. In particular, we have evaluated the effects of the symmetry and polarity of ambidentate donor subunits, differences in geometrical parameters (e.g., the size, angularity, and dimensionality) of Pt(II)-based acceptors and organic donors, the influence of temperature and solvent, and the effects of intermolecular steric interactions and hydrophobic interactions on self-organization. Our studies have shown that the extent of self-organization in the coordination-driven self-assembly of both 2D polygons and 3D polyhedra ranges from no organization (a statistical mixture of multiple products) to amplified organization (wherein a particular product or products are favored over others) and all the way to the absolute self-organization of discrete supramolecular assemblies. In many cases, inputs such as dipolar interactions, steric interactions, and differences in the geometric parameters of subunits, used either alone or as multiple factors simultaneously, can achieve absolute self-organization of discrete supramolecules. We have also observed instances where self-organization is not absolute and varies in its deviation from statistical results. Steric interactions are particularly useful control factors for driving such amplified self-organization because they can be subtly tuned through small structural variations. Having the ability to fully understand and control the self-organization of complex mixtures into specific synthetic supramolecules can provide a better understanding of analogous processes in biological systems. Furthermore, self-organization may allow for the facile synthesis of complex multifunctional, multicomponent systems from simply mixing a collection of much simpler, judiciously designed individual molecular components.
Figures





References
-
- Anderson L, Blomberg L, Flegel M, Lepsa L, Nilsson B, Verlander M. Large-scale Synthesis of Peptides. Biopolymers. 2000;55:227–250. - PubMed
-
- Zhang X, Houk KN. Why Enzymes are Proficient Catalysts: Beyond the Pauling Paradigm. Acc. Chem. Res. 2005;38:379–385. - PubMed
-
- Lindsey JS. Self-Assembly in Synthetic Routes to Molecular Devices. Biological Principles and Chemical Perspectives: a Review. New. J. Chem. 1991;15:153–180.
- Whitesides GM, Grzybowski B. Self-Assembly at all Scales. Science. 2002;295:2418–2421. - PubMed
-
- Lehn JM. New York: VCH; 1995. Supramolecular Chemistry: Concept and Perspectives.
- Matthew CT, Stoddart JF. Synthetic Supramolecular Chemistry. Acc. Chem. Res. 1997;30:393–401.
-
- Pederson CJ. Ger. Offen. 1970 DE 70-2015732.
- Cram DJ, Cram JM. Design of Complexes Between Synthetic Hosts and Organic Guests. Acc. Chem. Res. 1978;11:8–14.
- Lehn JM. Cryptates: the Chemistry of Macropolycyclic Inclusion Complexes. Acc. Chem. Res. 1978;11:49–57.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

