Processing difficulties and instability of carbohydrate microneedle arrays
- PMID: 19555249
- PMCID: PMC2900182
- DOI: 10.1080/03639040902882280
Processing difficulties and instability of carbohydrate microneedle arrays
Abstract
Background: A number of reports have suggested that many of the problems currently associated with the use of microneedle (MN) arrays for transdermal drug delivery could be addressed by using drug-loaded MN arrays prepared by moulding hot melts of carbohydrate materials.
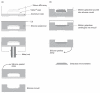
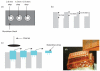
Methods: In this study, we explored the processing, handling, and storage of MN arrays prepared from galactose with a view to clinical application.
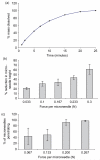
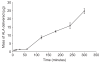
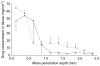
Results: Galactose required a high processing temperature (160 degrees C), and molten galactose was difficult to work with. Substantial losses of the model drugs 5-aminolevulinic acid (ALA) and bovine serum albumin were incurred during processing. While relatively small forces caused significant reductions in MN height when applied to an aluminium block, this was not observed during their relatively facile insertion into heat-stripped epidermis. Drug release experiments using ALA-loaded MN arrays revealed that less than 0.05% of the total drug loading was released across a model silicone membrane. Similarly, only low amounts of ALA (approximately 0.13%) and undetectable amounts of bovine serum albumin were delivered when galactose arrays were combined with aqueous vehicles. Microscopic inspection of the membrane following release studies revealed that no holes could be observed in the membrane, indicating that the partially dissolved galactose sealed the MN-induced holes, thus limiting drug delivery. Indeed, depth penetration studies into excised porcine skin revealed that there was no significant increase in ALA delivery using galactose MN arrays, compared to control (P value < 0.05). Galactose MNs were unstable at ambient relative humidities and became adhesive.
Conclusion: The processing difficulties and instability encountered in this study are likely to preclude successful clinical application of carbohydrate MNs. The findings of this study are of particular importance to those in the pharmaceutical industry involved in the design and formulation of transdermal drug delivery systems based on dissolving MN arrays. It is hoped that we have illustrated conclusively the difficulties inherent in the processing and storage of carbohydrate-based dissolving MNs and that those in the industry will now follow alternative approaches.
Figures



References
-
- Henry S, McAllister DV, Allen MG, Prausnitz MR. Microfabricated microneedles: A novel approach to transdermal drug delivery. J Pharm Sci. 1998;87:922–5. - PubMed
-
- Prausnitz MR. Microneedles for transdermal drug delivery. Adv Drug Deliv Rev. 2004;56:581–87. - PubMed
-
- Cormier M, Johnson B, Ameri M, Nyam K, Libiran L, Zhang DD. Transdermal delivery of desmopressin using a coated microneedle array patch system. J Control Release. 2004;97:503–11. - PubMed
-
- Coulman SA, Barrow D, Anstey A, Gateley C, Morrissey A, Wilke N. Minimally invasive cutaneous delivery of macromolecules and plasmid DNA via microneedles. Curr Drug Deliv. 2006;3:65–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
