Temporal changes in mouse brain fatty acid amide hydrolase activity
- PMID: 19555737
- PMCID: PMC2773183
- DOI: 10.1016/j.neuroscience.2009.06.043
Temporal changes in mouse brain fatty acid amide hydrolase activity
Abstract
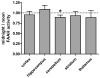
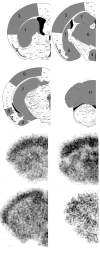
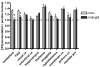
Fatty acid amide hydrolase (FAAH) activity is known to mediate the tone of endogenous fatty acid amides including the endocannabinoid anandamide. FAAH is a potential therapeutic target because genetic or pharmacological ablation of FAAH promotes analgesia and anxiolytic effects without disrupting motor coordination. Little is known about the endogenous temporal fluctuations of brain FAAH activity. This is the first comprehensive study examining temporal fluctuations in mouse brain FAAH activity. Regional mouse brain homogenates were generated at the midpoint of the light ("noon") and dark ("midnight") cycles. While immunoblots revealed no significant changes (P>0.05) in regional activity between these two time points, in vitro activity assays detected a subtle 10% reduction (P<0.05) in cerebellar FAAH activity at midnight. A novel ex vivo autoradiography technique permitted the study of 11 different brain regions, many of which cannot be studied using traditional in vitro methods. The cerebellum and the periaqueductal gray both exhibited significant (P<0.05) reductions in regional FAAH activity in "midnight" brains. These data confirm the need to account for temporal changes in FAAH activity when therapeutically targeting FAAH.
Figures


References
-
- Aviello G, Romano B, Izzo AA. Cannabinoids and gastrointestinal motility: animal and human studies. Eur Rev Med Pharmacol Sci. 2008;12(Suppl 1):81–93. - PubMed
-
- Cannon JT, Prieto GJ, Lee A, Liebeskind JC. Evidence for opioid and non-opioid forms of stimulation-produced analgesia in the rat. Brain Res. 1982;243:315–321. - PubMed
-
- Cota D. Role of the endocannabinoid system in energy balance regulation and obesity. Front Horm Res. 2008;36:135–145. - PubMed
-
- Day TA, Rakhshan F, Deutsch DG, Barker EL. Role of fatty acid amide hydrolase in the transport of the endogenous cannabinoid anandamide. Mol Pharmacol. 2001;59:1369–1375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

